Downloaded from the Worldclim Website ( Accessed on 5 January 2021)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecosystem Services Changes Between 2000 and 2015 in the Loess Plateau, China: a Response to Ecological Restoration
RESEARCH ARTICLE Ecosystem services changes between 2000 and 2015 in the Loess Plateau, China: A response to ecological restoration Dan Wu1, Changxin Zou1, Wei Cao2*, Tong Xiao3, Guoli Gong4 1 Nanjing Institute of Environmental Sciences, Ministry of Environmental Protection, Nanjing, China, 2 Key Laboratory of Land Surface Pattern and Simulation, Institute of Geographic Sciences and Natural Resources Research, CAS, Beijing, China, 3 Satellite Environment Center, Ministry of Environmental Protection, Beijing, China, 4 Shanxi Academy of Environmental Planning, Taiyuan, China a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract The Loess Plateau of China is one of the most severe soil and water loss areas in the world. Since 1999, the Grain to Green Program (GTGP) has been implemented in the region. This OPEN ACCESS study aimed to analyze spatial and temporal variations of ecosystem services from 2000 to Citation: Wu D, Zou C, Cao W, Xiao T, Gong G 2015 to assess the effects of the GTGP, including carbon sequestration, water regulation, (2019) Ecosystem services changes between 2000 soil conservation and sand fixation. During the study period, the area of forest land and and 2015 in the Loess Plateau, China: A response grassland significantly expanded, while the area of farmland decreased sharply. Ecosystem to ecological restoration. PLoS ONE 14(1): services showed an overall improvement with localized deterioration. Carbon sequestration, e0209483. https://doi.org/10.1371/journal. pone.0209483 water regulation and soil conservation increased substantially. Sand fixation showed a decreasing trend mainly because of decreased wind speeds. There were synergies Editor: Debjani Sihi, Oak Ridge National Laboratory, UNITED STATES between carbon sequestration and water regulation, and tradeoffs between soil conserva- tion and sand fixation. -

New Insight Into the Phylogeographic Pattern Of
New insight into the phylogeographic pattern of Liriodendron chinense (Magnoliaceae) revealed by chloroplast DNA: east–west lineage split and genetic mixture within western subtropical China Aihong Yang, Yongda Zhong, Shujuan Liu, Lipan Liu, Tengyun Liu, Yanqiang Li and Faxin Yu The Key Laboratory of Horticultural Plant Genetic and Improvement of Jiangxi, Institute of Biological Resources, Jiangxi Academy of Sciences, Nanchang, Jiangxi, China ABSTRACT Background: Subtropical China is a global center of biodiversity and one of the most important refugia worldwide. Mountains play an important role in conserving the genetic resources of species. Liriodendron chinense is a Tertiary relict tree largely endemic to subtropical China. In this study, we aimed to achieve a better understanding of the phylogeographical pattern of L. chinense andtoexploretheroleofmountainsintheconservationofL. chinense genetic resources. Methods: Three chloroplast regions (psbJ-petA, rpl32-ndhF, and trnK5’-matK) were sequenced in 40 populations of L. chinense for phylogeographical analyses. Relationships among chloroplast DNA (cpDNA) haplotypes were determined using median-joining networks, and genetic structure was examined by spatial analysis of molecular variance (SAMOVA). The ancestral area of the species was reconstructed using the Bayesian binary Markov Chain Monte Carlo (BBM) method according to its geographic distribution and a maximum parsimony (MP) tree based on Bayesian methods. Results: Obvious phylogeographic structure was found in L. chinense. SAMOVA Submitted 13 September 2018 revealed seven groups matching the major landscape features of the L. chinense Accepted 26 December 2018 Published 1 February 2019 distribution area. The haplotype network showed three clades distributed in the eastern, southwestern, and northwestern regions. Separate northern and southern Corresponding author Faxin Yu, [email protected] refugia were found in the Wu Mountains and Yungui Plateau, with genetic admixture in the Dalou Mountains and Wuling Mountains. -

China's Special Poor Areas and Their Geographical Conditions
sustainability Article China’s Special Poor Areas and Their Geographical Conditions Xin Xu 1,2, Chengjin Wang 1,2,*, Shiping Ma 1,2 and Wenzhong Zhang 1,2 1 Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China; [email protected] (X.X.); [email protected] (S.M.); [email protected] (W.Z.) 2 College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] Abstract: Special functional areas and poor areas tend to spatially overlap, and poverty is a common feature of both. Special poor areas, taken as a kind of “policy space,” have attracted the interest of researchers and policymakers around the world. This study proposes a basic concept of special poor areas and uses this concept to develop a method to identify them. Poor counties in China are taken as the basic research unit and overlaps in spatial attributes including old revolutionary bases, borders, ecological degradation, and ethnic minorities, are used to identify special poor areas. The authors then analyze their basic quantitative structure and pattern of distribution to determine the geographical bases’ formation and development. The results show that 304 counties in China, covering a vast territory of 12 contiguous areas that contain a small population, are lagging behind the rest of the country. These areas are characterized by rich energy and resource endowments, important ecological functions, special historical status, and concentrated poverty. They are considered “special poor” for geographical reasons such as a relatively harsh natural geographical environment, remote location, deteriorating ecological environment, and an inadequate infrastructure network and public service system. -

Thermogenesis, Flowering and the Association with Variation in Floral Odour Attractants in Magnolia Sprengeri (Magnoliaceae)
Thermogenesis, Flowering and the Association with Variation in Floral Odour Attractants in Magnolia sprengeri (Magnoliaceae) Ruohan Wang1, Sai Xu1,3, Xiangyu Liu2, Yiyuan Zhang1, Jianzhong Wang1, Zhixiang Zhang2* 1 National Engineering Laboratory for Tree Breeding, Key Laboratory for Genetics and Breeding of Forest Trees and Ornamental Plants, Ministry of Education, College of Biological Sciences and Biotechnology, Beijing Forestry University,Beijing, China, 2 Lab of Systematic Evolution and Biogeography of Woody Plants, College of Nature Conservation, Beijing Forestry University,Beijing, China, 3 School of Environment, Tsinghua University, Beijing, China Abstract Magnolia sprengeri Pamp. is an ornamentally and ecologically important tree that blooms at cold temperatures in early spring. In this study, thermogenesis and variation in the chemical compounds of floral odours and insect visitation in relation to flowering cycles were studied to increase our understanding of the role of floral thermogenesis in the pollination biology of M. sprengeri. There were five distinct floral stages across the floral cycle of this species: pre-pistillate, pistillate, pre- staminate, staminate and post-staminate. Floral thermogenesis during anthesis and consisted of two distinct peaks: one at the pistillate stage and the other at the staminate stage. Insects of five families visited M. sprengeri during the floral cycle, and sap beetles (Epuraea sp., Nitidulidae) were determined to be the most effective pollinators, whereas bees (Apis cerana, Apidae) were considered to be occasional pollinators. A strong fragrance was released during thermogenesis, consisting of 18 chemical compounds. Although the relative proportions of these compounds varied at different floral stages across anthesis, linalool, 1-iodo-2-methylundecane and 2,2,6-trimethyl-6-vinyltetrahydro-2H-pyran-3-ol were dominant. -

(Leech, 1890) (Lepidoptera: Hesperiidae) with Description of Female Genitalia and Taxonomic Notes
© Entomologica Fennica. 31 August 2016 Distribution of Onryza maga (Leech, 1890) (Lepidoptera: Hesperiidae) with description of female genitalia and taxonomic notes Guoxi Xue, Yufei Li, Zihao Liu, Meng Li & Yingdang Ren Xue, G. X., Li, Y.F., Liu, Z. H., Li, M. & Ren, Y.D. 2016: Distribution of Onryza maga (Leech, 1890) (Lepidoptera: Hesperiidae) with description of female geni- talia and taxonomic notes. — Entomol. Fennica 27: 70–76. For more than twenty years, Hainan, Vietnam, Myanmar, Thailand, Malaysia, Singapore and Indonesia have been erroneously reported in Chinese literature as belonging to the distribution range of Onryza maga (Leech 1890). Based upon a careful survey of specimens and relevant literature, these regions are omitted from the known range of this species. Onryza maga maga is found from northeast Guizhou, south Henan and Qinling-Daba Mountains in Shaanxi of China, its oc- currence in Hunan is confirmed. The adults are redescribed and the variability of wing patterns is discussed. Female genitalia are illustrated and described for the first time. Some biological information and an updated distribution map of the species are provided. G. X. Xue & M. Li, School of Food and Bioengineering, Zhengzhou University of Light Industry, No. 5 Dongfeng Road, Zhengzhou, Henan, 450002, P. R. China; Corresponding author’s e-mail: [email protected] Y. F. Li, School of Medicine, Xi’an Jiaotong University, No. 76 Yanta West Road, Xi’an, Shaanxi, 710061, P. R. China Z. H. Liu, School of Physics, University of Science and Technology of China, No. 96 Jinzhai Road, Hefei, Anhui, 230026, P. R. China Y. D. -

Study on the Coniferous Characters of Pinus Yunnanensis and Its Clustering Analysis
Journal of Polymer Science and Engineering (2017) Original Research Article Study on the Coniferous Characters of Pinus yunnanensis and Its Clustering Analysis Zongwei Zhou,Mingyu Wang,Haikun Zhao Huangshan Institute of Botany, Anhui Province, China ABSTRACT Pine is a relatively easy genus for intermediate hybridization. It has been widely believed that there should be a natural hybrid population in the distribution of Pinus massoniona Lamb. and Pinus hangshuanensis Hsia, that is, the excessive type of external form between Pinus massoniana and Pinus taiwanensis exist. This paper mainly discusses the traits and clustering analysis of coniferous lozeng in Huangshan scenic area. This study will provide a theoretical basis for the classification of long and outstanding Huangshan Song and so on. At the same time, it will provide reference for the phenomenon of gene seepage between the two species. KEYWORDS: Pinus taiwanensis Pinus massoniana coniferous seepage clustering Citation: Zhou ZW, Wang MY, ZhaoHK, et al. Study on the Coniferous Characters of Pinus yunnanensis and Its Clustering Analysis, Gene Science and Engineering (2017); 1(1): 19–27. *Correspondence to: Haikun Zhao, Huangshan Institute of Botany, Anhui Province, China, [email protected]. 1. Introduction 1.1. Research background Huangshan Song distribution in eastern China’s subtropical high mountains, more than 700m above sea level. Masson pine is widely distributed in the subtropical regions of China, at the lower reaches of the Yangtze River, vertically distributed below 700m above sea level, the upper reaches of the Yangtze River area, the vertical height of up to 1200 - 1500m or so. In the area of Huangshan Song and Pinus massoniana, an overlapping area of Huangshan Song and Pinus massoniana was formed between 700 - 1000m above sea level. -

Preparing the Shaanxi-Qinling Mountains Integrated Ecosystem Management Project (Cofinanced by the Global Environment Facility)
Technical Assistance Consultant’s Report Project Number: 39321 June 2008 PRC: Preparing the Shaanxi-Qinling Mountains Integrated Ecosystem Management Project (Cofinanced by the Global Environment Facility) Prepared by: ANZDEC Limited Australia For Shaanxi Province Development and Reform Commission This consultant’s report does not necessarily reflect the views of ADB or the Government concerned, and ADB and the Government cannot be held liable for its contents. (For project preparatory technical assistance: All the views expressed herein may not be incorporated into the proposed project’s design. FINAL REPORT SHAANXI QINLING BIODIVERSITY CONSERVATION AND DEMONSTRATION PROJECT PREPARED FOR Shaanxi Provincial Government And the Asian Development Bank ANZDEC LIMITED September 2007 CURRENCY EQUIVALENTS (as at 1 June 2007) Currency Unit – Chinese Yuan {CNY}1.00 = US $0.1308 $1.00 = CNY 7.64 ABBREVIATIONS ADB – Asian Development Bank BAP – Biodiversity Action Plan (of the PRC Government) CAS – Chinese Academy of Sciences CASS – Chinese Academy of Social Sciences CBD – Convention on Biological Diversity CBRC – China Bank Regulatory Commission CDA - Conservation Demonstration Area CNY – Chinese Yuan CO – company CPF – country programming framework CTF – Conservation Trust Fund EA – Executing Agency EFCAs – Ecosystem Function Conservation Areas EIRR – economic internal rate of return EPB – Environmental Protection Bureau EU – European Union FIRR – financial internal rate of return FDI – Foreign Direct Investment FYP – Five-Year Plan FS – Feasibility -

GIS Assessment of the Status of Protected Areas in East Asia
CIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, 1. Lysenko, S. Chape, I. May and C. Brown March 2005 IUCN V 9> m The World Conservation Union UNEP WCMC Digitized by the Internet Archive in 20/10 with funding from UNEP-WCMC, Cambridge http://www.archive.org/details/gisassessmentofs05mack GIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, I. Lysenko, S. Chape, I. May and C. Brown March 2005 UNEP-WCMC IUCN - The World Conservation Union The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of UNEP, UNEP-WCMC, and IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. UNEP-WCMC or its collaborators have obtained base data from documented sources believed to be reliable and made all reasonable efforts to ensure the accuracy of the data. UNEP-WCMC does not warrant the accuracy or reliability of the base data and excludes all conditions, warranties, undertakings and terms express or implied whether by statute, common law, trade usage, course of dealings or otherwise (including the fitness of the data for its intended use) to the fullest extent permitted by law. The views expressed in this publication do not necessarily reflect those of UNEP, UNEP-WCMC, and IUCN. Produced by: UNEP World Conservation Monitoring Centre and IUCN, Gland, Switzerland and Cambridge, UK Cffti IUCN UNEP WCMC The World Conservation Union Copyright: © 2005 UNEP World Conservation Monitoring Centre Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. -

Hubei Shennongjia
ASIA / PACIFIC HUBEI SHENNONGJIA CHINA Laojunshan Component of the property - © IUCN Bruce Jefferies China - Hubei Shennongjia WORLD HERITAGE NOMINATION – IUCN TECHNICAL EVALUATION HUBEI SHENNONGJIA (CHINA) – ID 1509 IUCN RECOMMENDATION TO WORLD HERITAGE COMMITTEE: To inscribe the property under natural criteria. Key paragraphs of Operational Guidelines: Paragraph 77: Nominated property meets World Heritage criteria. Paragraph 78: Nominated property meets integrity and protection and management requirements. 1. DOCUMENTATION S. and Hong Qian. Global Significance of Plant Diversity in China. In The Plants of China: A a) Date nomination received by IUCN: 16 March Companion to the Flora of China (2015). Huang, J. H., 2015 Chen, J.H., Ying, J.S., and Ke‐Ping M. Features and distribution patterns of Chinese endemic seed plant b) Additional information officially requested from species. Journal of Systematics and Evolution 49, no. and provided by the State Party: On 6 September 2 (2011): 81-94. Li, Y. (2004). The effect of forest 2015, the State Party responded to issues which arose clear-cutting on habitat use in Sichuan snub-nosed during the course of the IUCN field evaluation mission. monkey (Rhinopithecus roxellana) in Shennongjia The letter, with accompanying maps, addressed a Nature Reserve, China. Primates 45.1 69-72.. López- range of issues and confirmed extensions to the Pujol, J., et al. (2011). Mountains of Southern China as nominated area and buffer zone in the Badong County “plant museums” and “plant cradles”: evolutionary and area. Following the IUCN World Heritage Panel a conservation insights. Mountain Research and progress report was sent to the State Party on 16 Development,31(3), 261-269. -
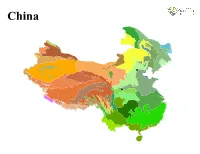
R Graphics Output
China China LEGEND Previously sampled Malaise trap site Ecoregion Alashan Plateau semi−desert North Tibetan Plateau−Kunlun Mountains alpine desert Altai alpine meadow and tundra Northeast China Plain deciduous forests Altai montane forest and forest steppe Northeast Himalayan subalpine conifer forests Altai steppe and semi−desert Northern Indochina subtropical forests Amur meadow steppe Northern Triangle subtropical forests Bohai Sea saline meadow Northwestern Himalayan alpine shrub and meadows Central China Loess Plateau mixed forests Nujiang Langcang Gorge alpine conifer and mixed forests Central Tibetan Plateau alpine steppe Ordos Plateau steppe Changbai Mountains mixed forests Pamir alpine desert and tundra Changjiang Plain evergreen forests Qaidam Basin semi−desert Da Hinggan−Dzhagdy Mountains conifer forests Qilian Mountains conifer forests Daba Mountains evergreen forests Qilian Mountains subalpine meadows Daurian forest steppe Qin Ling Mountains deciduous forests East Siberian taiga Qionglai−Minshan conifer forests Eastern Gobi desert steppe Rock and Ice Eastern Himalayan alpine shrub and meadows Sichuan Basin evergreen broadleaf forests Eastern Himalayan broadleaf forests South China−Vietnam subtropical evergreen forests Eastern Himalayan subalpine conifer forests Southeast Tibet shrublands and meadows Emin Valley steppe Southern Annamites montane rain forests Guizhou Plateau broadleaf and mixed forests Suiphun−Khanka meadows and forest meadows Hainan Island monsoon rain forests Taklimakan desert Helanshan montane conifer forests -

Atlanticrhodo
AtlanticRhodo www.AtlanticRhodo.org Volume 30: Number 1 February 2006 February 2006 1 Positions of Responsibility 2005 - 2006 President Penny Gael 826-2440 Director - Social Sandy Brown 683-2615 Vice-President Ruth Jackson 454-4861 Director - R.S.C. Horticulture Audrey Fralic 683-2711 (National) Rep. Sheila Stevenson 479-3740 Director Cora Swinamer 826-7705 Secretary Lyla MacLean 466-4490 Newsletter Mary Helleiner 429-0213 Treasurer Chris Hopgood 479-0811 Website King Butler 542-5805 Membership Betty MacDonald 852-2779 Library Elizabeth Naylor 429-0557 Past President Sheila Stevenson 479-3740 Seed Exchange Sharon Bryson 863-6307 Director - Education Jenny Sandison 624-9013 May - Advance Plant Sale Ken Shannik 422-2413 Director - Communications Mary Helleiner 429-0213 May- Public Plant Sale Duff & Donna Evers 835-2586 Membership Atlantic Rhododendron & Horticultural Society. Fees are $ 15.00 per year, due January 1, 2006. Make cheques payable to Atlantic Rhododendron and Horticultural Society. ARHS is a chapter in District 12 of the American Rhododendron Society. For benefits see ARHS website www.atlanticrhodo.org American Rhododendron Society Fees are $50.00 Canadian per year and include ARHS membership. Make cheques payable to American Rhododendron Society District 12. For benefits see www.rhododendron.org Cheques should be sent to ARHS Membership Secretary, Betty MacDonald , 534 Prospect Road, Prospect Bay NS B3T1Z8 Please include name, address with postal code, e-mail address and telephone number (for organizational purposes). AtlanticRhodo is the Newsletter of the Atlantic Rhododendron and Horticultural Society. We welcome your comments, suggestions, articles, photos and other material for publication. Send all material to the editor. -

Characterization of Two PEBP Genes, Srft and Srmft, in Thermogenic
www.nature.com/scientificreports OPEN Characterization of two PEBP genes, SrFT and SrMFT, in thermogenic skunk cabbage Received: 09 March 2016 Accepted: 20 June 2016 (Symplocarpus renifolius) Published: 08 July 2016 Yasuko Ito-Inaba1, Hiromi Masuko-Suzuki2, Haruhiko Maekawa1,3, Masao Watanabe2 & Takehito Inaba3 Floral thermogenesis has been found in dozens of primitive seed plants and the reproductive organs in these plants produce heat during anthesis. Thus, characterization of the molecular mechanisms underlying flowering is required to fully understand the role of thermogenesis, but this aspect of thermogenic plant development is largely unknown. In this study, extensive database searches and cloning experiments suggest that thermogenic skunk cabbage (Symplocarpus renifolius), which is a member of the family Araceae, possesses two genes encoding phosphatidyl ethanolamine-binding proteins (PEBP), FLOWERING LOCUS T (SrFT) and MOTHER OF FT AND TFL1 (SrMFT). Functional analyses of SrFT and SrMFT in Arabidopsis indicate that SrFT promotes flowering, whereasSrMFT does not. In S. renifolius, the stage- and tissue-specific expression ofSrFT was more evident than that of SrMFT. SrFT was highly expressed in flowers and leaves and was mainly localized in fibrovascular tissues. In addition, microarray analysis revealed that, within floral tissues,SrFT was co-regulated with the genes associated with cellular respiration and mitochondrial function, including ALTERNATIVE OXIDASE gene proposed to play a major role in floral thermogenesis. Taken together, these data suggest that, among the PEBP genes, SrFT plays a role in flowering and floral development in the thermogenic skunk cabbage. Floral thermogenesis has been found in dozens of flowering plants including basal angiosperms (Nymphaeaceae and Schisandraceae)1–4, magnoliids (Magnoliaceae)5–7, monocots (Araceae)8–10, and eudicots (Nelumbonaceae and Rafflesiaceae)10,11.