An Arbitrary-Spectrum Spatial Visual Stimulator for Vision Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Digital Driving Technique for an 8 B QVGA AMOLED Display Using Modulation Jang Jae Hyuk
Purdue University Purdue e-Pubs Department of Electrical and Computer Department of Electrical and Computer Engineering Faculty Publications Engineering January 2009 A digital driving technique for an 8 b QVGA AMOLED display using modulation Jang Jae Hyuk Kwon Minho E. Tjandranegara Lee Kywro Jung Byunghoo Follow this and additional works at: http://docs.lib.purdue.edu/ecepubs Jae Hyuk, Jang; Minho, Kwon; Tjandranegara, E.; Kywro, Lee; and Byunghoo, Jung, "A digital driving technique for an 8 b QVGA AMOLED display using modulation" (2009). Department of Electrical and Computer Engineering Faculty Publications. Paper 25. http://dx.doi.org/http://dx.doi.org/10.1109/ISSCC.2009.4977412 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Please click on paper title to view Visual Supplement. ISSCC 2009 / SESSION 15 / DISPLAY AND IMAGER ELECTRONICS / 15.4 15.4 A Digital Driving Technique for an 8b QVGA AMOLED A 1st-order ΔΣ modulator is used because the human visual system is closely Display Using ΔΣ Modulation related to a 2nd-order LPF. Figure 15.4.4 shows the simulated resolutions and gate scan times in the face Jae Hyuk Jang1, Minho Kwon1, Edwin Tjandranegara1, Kywro Lee2, 1 of different OSR values. The resolutions are estimated by measuring the peak Byunghoo Jung output SNRs with triangle-wave inputs. The gate scan time is the time period allowed for charging and discharging each scan line for the minimum-length 1 Purdue University, West Lafayette, IN sub-field, and calculated for the (320×240×RGB) QVGA panel with 2-TFT-1- 2LG Electronics, Seoul, Korea Cap pixels. -

Cathode-Ray Tube Displays for Medical Imaging
DIGITAL IMAGING BASICS Cathode-Ray Tube Displays for Medical Imaging Peter A. Keller This paper will discuss the principles of cathode-ray crease the velocity of the electron beam for tube displays in medical imaging and the parameters increased light output from the screen; essential to the selection of displays for specific 4. a focusing section to bring the electron requirements. A discussion of cathode-ray tube fun- beam to a sharp focus at the screen; damentals and medical requirements is included. 9 1990bu W.B. Saunders Company. 5. a deflection system to position the electron beam to a desired location on the screen or KEY WORDS: displays, cathode ray tube, medical scan the beam in a repetitive pattern; and irnaging, high resolution. 6. a phosphor screen to convert the invisible electron beam to visible light. he cathode-ray tube (CRT) is the heart of The assembly of electrodes or elements mounted T almost every medical display and its single within the neck of the CRT is commonly known most costly component. Brightness, resolution, as the "electron gun" (Fig 2). This is a good color, contrast, life, cost, and viewer comfort are analogy, because it is the function of the electron gun to "shoot" a beam of electrons toward the all strongly influenced by the selection of a screen or target. The velocity of the electron particular CRT by the display designer. These beam is a function of the overall accelerating factors are especially important for displays used voltage applied to the tube. For a CRT operating for medical diagnosis in which patient safety and at an accelerating voltage of 20,000 V, the comfort hinge on the ability of the display to electron velocity at the screen is about present easily readable, high-resolution images 250,000,000 mph, or about 37% of the velocity of accurately and rapidly. -

A Rectangular Area Filling Display System Architecture Daniel S
A Rectangular Area Filling Display System Architecture Daniel S. Whelan Computer Science Department California I nstitute of Technology 4716 :TM: 82 A Rectangulr:.r Area Filling Display System Ar.:hltecture Daniel So Whelan California Institute of Technology Pasadena, California 91125 Technical Memo #4716 A Reetangular Area Filling Display System Arehiteeture Daniel S. Whelan Computer Science Department California Institute of Technology Abstraet A display system architecture which has rectangular area filling as its primitive operation is presented. It is shown that lines can be drawn significantly faster while rendition of filled boxes 2 shows an O(n ) speed improvement. Furthermore filled polygons can be rendered with an O(n) speed improvement. Implementation of this rectangular area filling architecture is discussed and refined. A custom VLSI integrated circuit is currently being designed to implement this rectangular area filling architecure and at the same time reduce the display memory system video refresh bandwidth requirements. Introduetion Presently, most computer graphics systems a.re of two architectures. Calligraphic displays render lines a.s their primitive operation whereas raster scan displays render pixels as their primitives. This paper discusses a new type of display that renders filled rectangular areas as its primitive operation. This type of display can be considered a sub-class of raster scan displays and as such it retains all of the attributes of such display systems. This paper examines algorithms for rendering lines, filled boxes and filled polygons and compares time complexities for these algorithms on conventional raster scan displays with those on rectangular area filling displays. This analysis will show that the design and implementation of a rectangular area filling display system is desirable. -

360 PPI Flip-Chip Mounted Active Matrix Addressable Light Emitting
678 JOURNAL OF DISPLAY TECHNOLOGY, VOL. 9, NO. 8, AUGUST 2013 360 PPI Flip-Chip Mounted Active Matrix Addressable Light Emitting Diode on Silicon (LEDoS) Micro-Displays Zhao Jun Liu, Wing Cheung Chong, Ka Ming Wong, and Kei May Lau, Fellow, IEEE Abstract—In this paper, we describe the design and fabrication and excellent visibility under bright daylight. Passive matrix-ad- of 360 PPI flip-chip mounted active matrix (AM) addressable dressable LED micro-displays on sapphire substrates have been light emitting diode on silicon (LEDoS) micro-displays. The reported [9]–[12]. The dimensions and pixel brightness in pas- LEDoS micro-displays are self-emitting devices which have higher light efficiency than liquid crystal based displays (LCDs) and sive addressable LED micro-displays are limited by the loading longer lifetime than organic light emitting diodes (OLEDs) based effect because the LED pixels in the same row or column share displays. The LEDoS micro-displays were realized by integrating the fixed driving current of the periphery driving supplies. The monolithic LED micro-arrays and silicon-based integrated circuit power efficiency of passive matrix LED micro-displays was using a flip-chip bonding technique. The active matrix driving also low because of parasitic resistance and capacitance with scheme was designed on the silicon to provide sufficient driving current and individual controllability of each LED pixel. Red, a large amount of connecting wires. LED arrays addressed by green, blue and Ultraviolet (UV) LEDoS micro-displays with a a single transistor with epitaxial stack method were also de- pixel size of 50 m and pixel pitch of 70 m were demonstrated. -

Graphics Systems
Graphics Systems Dr. S.M. Malaek Assistant: M. Younesi Overview Display Hardware How are images displayed? Overview (Display Devices) Raster Scan Displays Random Scan Displays Color CRT Monirors Direct View Storage Tube Flat panel Displays Three Dimensional Viewing Devices Stereoscopic and Virtual Reality System Overview (Display Devices) The display systems are often referred to as Video Monitor or Video Display Unit (VDU). Display Hardware Video Display Devices The primary output device in a graphics system is a monitor. Video Monitor Cathode Ray Tube (CRT) 1. Electron Guns 2. Electron Beams 3. Focusing Coils 4. Deflection Coils 5. Anode Connection 6. Shadow Mask 7. Phosphor layer 8. Close-up of the phosphor coated inner side of the screen Cathode Ray Tube (CRT) Refresh CRT Light emitted by the Phosphor fades very rapidly. Refresh CRT: One way to keep the phosphor glowing is to redraw the picture repeatedly by quickly directing the electron beam back over the same points. Electron Gun Electron Gun Heat is supplied to the cathode by the filament. Electron Gun The free electrons are then accelerated toward the phosphor coating by a high positive voltage. High Positive Voltage A positively charged metal coating on the inside of the CRT envelope near the phosphor screen. A positively charged metal High Positive Voltage An accelerating anode . Electron Gun Intensity of the electron beam is controlled by setting voltage level on the control grid. Electron Gun A smaller negative voltage on the control grid simply decrease the number of electrons passing through. Focusing System Focusing System The focusing system is needed to force the electron beam to converge into a small spot as it strikes the phosphor. -

Transparent Active Matrix Organic Light-Emitting Diode Displays
NANO LETTERS 2008 Transparent Active Matrix Organic Vol. 8, No. 4 Light-Emitting Diode Displays Driven by 997-1004 Nanowire Transistor Circuitry Sanghyun Ju,†,§ Jianfeng Li,£ Jun Liu,£ Po-Chiang Chen,‡ Young-geun Ha,£ Fumiaki Ishikawa,‡ Hsiaokang Chang,‡ Chongwu Zhou,‡ Antonio Facchetti,£ David B. Janes,*,† and Tobin J. Marks*,£ School of Electrical and Computer Engineering, and Birck Nanotechnology Center, Purdue UniVersity, West Lafayette, Indiana 47907, Department of Electrical Engineering, UniVersity of Southern California, Los Angeles, California 90089, and Department of Chemistry and the Materials Research Center, and the Institute for Nanoelectronics and Computing, Northwestern UniVersity, EVanston, Illinois 60208-3113 Received October 3, 2007 ABSTRACT Optically transparent, mechanically flexible displays are attractive for next-generation visual technologies and portable electronics. In principle, organic light-emitting diodes (OLEDs) satisfy key requirements for this applicationstransparency, lightweight, flexibility, and low-temperature fabrication. However, to realize transparent, flexible active-matrix OLED (AMOLED) displays requires suitable thin-film transistor (TFT) drive electronics. Nanowire transistors (NWTs) are ideal candidates for this role due to their outstanding electrical characteristics, potential for compact size, fast switching, low-temperature fabrication, and transparency. Here we report the first demonstration of AMOLED displays driven exclusively by NW electronics and show that such displays can be optically -

US 2007/0024547 A1 Jang Et Al
US 2007.0024547A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0024547 A1 Jang et al. (43) Pub. Date: Feb. 1, 2007 (54) ORGANIC LIGHT EMITTING DIODE (52) U.S. Cl. ................................................................ 345/81 DISPLAY DEVICE AND A DRIVING METHOD THEREOF (76) Inventors: Jin Jang, Seoul (KR); Ji-Ho Hur, (57) ABSTRACT Seoul (KR); Se-Hwan Kim, Seoul (KR); Youn-Duck Nam, Seoul (KR) Correspondence Address: Disclosed are an Organic Light Emitting Diode (OLED) DALY, CROWLEY, MOFFORD & DURKEE, display device having a pixel circuit which use a thin film LLP SUTE 301A transistor (TFT) as an active device and a driving method 354ATURNPIKE STREET thereof. The OLED display device can constantly obtain CANTON, MA 02021-2714 (US) luminance of the light emitting elements by elapsed time, because the brightness of the pixel for the signal Voltage is (21) Appl. No.: 11/396,925 not varied by a characteristic variance of the transistor (e.g., a driving element) and the OLED. Accordingly, the OLED (22) Filed: Apr. 3, 2006 display device according to the present invention can mini (30) Foreign Application Priority Data mizes the variance of the pixel brightness due to deteriora tion of the transistor and the OLED caused by usage for a Jul. 27, 2005 (KR)............................ 10-2005-0068514 long time and increase life span of the display device. Publication Classification Further, the OLED display device can display high quality of the image even in case of the high precision display, (51) Int. Cl. because it is controlled to flow the current to OLED included G09G 3/30 (2006.01) in each pixel. -

Graphics Hardware Cathode Ray Tube (CRT) Color CRT
Graphics Hardware Cathode Ray Tube (CRT) 1 2 3 4 5 6 Monitor (CRT, LCD,…) Graphics accelerator Scan controller Video Memory (frame buffer) 1. Filament (generate heat) Display/Graphics Processor 2. Cathode (emit electrons) CPU/Memory/Disk … 3. Control grid (control intensity) 4. Focus 5. Deflector 6. Phosphor coating Color CRT LED 3 electron guns, 3 color phosphor dots at each pixel Color = (red, green, blue) Black = (0,0,0) White = (1,1,1) Red = 0 to 100% Red = (1,0,0) Green = 0 to 100% Green = (0,1,0) Blue = 0 to 100% Blue = (0,0,1) … 1 LCD Plasma Panels Raster Display graphics How to draw a picture? Digital Display Based on (analog) raster-scan TV technology The screen (and a picture) consists of discrete pixels, and each pixel has one or multiple phosphor dots We have only one electron gun but many pixels in a picture need to be lit simultaneously… 2 Refresh Random Scan Order Refresh – the electron gun needs to come back to Old way: No pixels - The electron gun hit the pixel again before it fades out draws straight lines from location to An appropriate fresh rate depends on the property of location on the screen (vector graphics) phosphor coating Phosphor persistence: the time it takes for the a.k.a. calligraphic display, emitted light to decay to 1/10 of the original intensity Random scan device, vector drawing display Typical refresh rate: 60 – 80 times per second (Hz) Use either display list or (What will happen if refreshing is too slow or too storage tube technology fast?) Raster Scan Order Raster Scan Order What we do now: the electron gun will The electron gun will scan through the scan through the pixels from left to pixels from left to right, top to bottom right, top to bottom (scanline by (scanline by scanline) scanline) Horizontal retrace 3 Raster Scan Order Progressive vs. -
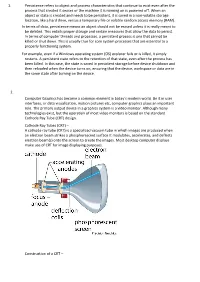
1. Persistence Refers to Object and Process Characteristics
1. Persistence refers to object and process characteristics that continue to exist even after the process that created it ceases or the machine it is running on is powered off. When an object or state is created and needs to be persistent, it is saved in a non-volatile storage location, like a hard drive, versus a temporary file or volatile random access memory (RAM). In terms of data, persistence means an object should not be erased unless it is really meant to be deleted. This entails proper storage and certain measures that allow the data to persist. In terms of computer threads and processes, a persistent process is one that cannot be killed or shut down. This is usually true for core system processes that are essential to a properly functioning system. For example, even if a Windows operating system (OS) explorer fails or is killed, it simply restarts. A persistent state refers to the retention of that state, even after the process has been killed. In this case, the state is saved in persistent storage before device shutdown and then reloaded when the device turns on, ensuring that the device, workspace or data are in the same state after turning on the device. 2. Computer Graphics has become a common element in today’s modern world. Be it in user interfaces, or data visualization, motion pictures etc, computer graphics plays an important role. The primary output device in a graphics system is a video monitor. Although many technologies exist, but the operation of most video monitors is based on the standard Cathode Ray Tube (CRT) design. -
![Review of Fast Methods for Point-Based Computer-Generated Holography [Invited]](https://docslib.b-cdn.net/cover/8855/review-of-fast-methods-for-point-based-computer-generated-holography-invited-2468855.webp)
Review of Fast Methods for Point-Based Computer-Generated Holography [Invited]
Review Vol. 6, No. 9 / September 2018 / Photonics Research 837 Review of fast methods for point-based computer-generated holography [Invited] 1, 2 1 P. W. M. T SANG, * T.-C. POON, AND Y. M. W U 1Department of Electronic Engineering, City University of Hong Kong, Hong Kong SAR, China 2Bradley Department of Electrical and Computer Engineering, Virginia Tech, Blacksburg, Virginia 24061, USA *Corresponding author: [email protected] Received 8 May 2018; revised 29 June 2018; accepted 2 July 2018; posted 6 July 2018 (Doc. ID 331162); published 7 August 2018 Computer-generated holography (CGH) is a technique for converting a three-dimensional (3D) object scene into a two-dimensional (2D), complex-valued hologram. One of the major bottlenecks of CGH is the intensive com- putation that is involved in the hologram generation process. To overcome this problem, numerous research works have been conducted with the aim of reducing arithmetic operations involved in CGH. In this paper, we shall review a number of fast CGH methods that have been developed in the past decade. These methods, which are commonly referred to as point-based CGH, are applied to compute digital Fresnel holograms for an object space that is represented in a point cloud model. While each method has its own strength and weakness, trading off conflicting issues, such as computation efficiency and memory requirement, they also exhibit potential grounds of synergy. We hope that this paper will bring out the essence of each method and provide some insight on how different methods may crossover into better ones. © 2018 Chinese Laser Press OCIS codes: (090.1760) Computer holography; (090.1995) Digital holography. -

Scanned Laser Displays for Head Mounted Displays Douglas E
Scanned Laser Displays for Head Mounted Displays Douglas E. Holmgren Department of Physics and Astronomy Warren Robinett Department of Computer Science University of North Carolina, Chapel Hill, NC Abstract Technologies applicable toward a display system in which a laser is raster scanned on the viewer's retina are reviewed. The properties of laser beam propagation and the inherent resolution of a laser scanning system are discussed. Scanning techniques employing rotating mirrors, galvonometer scanners, acousto-optic deflectors, and piezo-electric deflectors are described. Resolution, speed, deflection range, and physical size are strongly coupled properties of these technologies. For head-mounted display applications, a monochromatic system employing a laser diode source with acousto-optic and galvonometer scanners is deemed most practical. A resolution of 1000 x 1000 pixels at 60 frames per second should be possible with such a sytem. A radiometric analysis indicates that eye safety would not be a problem in a retina-scanning system. Introduction The recent interest in incorporating display devices into Head-Mounted displays (HMDs) imposes new requirements on display devices, particularly in terms of resolution, weight and volume. While small CRT and LCD displays exist, a 1-inch, 1000-line color CRT or LCD display is not currently available. Poor resolution is currently one of the major technical problems of HMDs, and this provides incentive to consider alternative display technologies that may be superior to CRTs and LCDs for use in HMDs. The Private Eye1 has demonstrated that a small, lightweight, medium-resolution monochromatic display can be built by modulating the elements of a 1-dimensional LED array and using a moving mirror to sweep out a 2D virtual image. -

Microcrystalline Silicon Based Thin Film Transistors Fabricated on Flexible Substrate Hanpeng Dong
Microcrystalline silicon based thin film transistors fabricated on flexible substrate Hanpeng Dong To cite this version: Hanpeng Dong. Microcrystalline silicon based thin film transistors fabricated on flexible substrate. Electronics. Université Rennes 1, 2015. English. NNT : 2015REN1S173. tel-02384928 HAL Id: tel-02384928 https://tel.archives-ouvertes.fr/tel-02384928 Submitted on 28 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° d’ordre : ANNÉE 2015 THÈSE / UNIVERSITÉ DE RENNES 1 sous le sceau de l’Université Européenne de Bretagne pour le grade de DOCTEUR DE L’UNIVERSITÉ DE RENNES 1 Mention : Electronique Ecole doctorale MATISSE présentée par Hanpeng Dong Préparée UMR-CNRS 6164 IETR Institut d’Electronique et de Télécommunications de Rennes UFR ISTIC Thèse soutenue à Rennes Intitulé de la thèse : le 25 septembre 2015 Microcrystalline devant le jury composé de : Yvan BONNASSIEUX silicon based Thin Professeur, Ecole Polytechnique Palaiseau / rapporteur Film Transistors François TEMPLIER Ingénieur HDR CEA-LETI MINATEC / rapporteur fabricated on Flexible Takashi NOGUCHI Professeur, Université Okinawa Japon / Substrate examinateur Lei WEI Professeur, Université South-East Nanjing Chine / examinateur Emmanuel JACQUES Maître de Conférences Université Rennes 1 / examinateur Tayeb MOHAMMED-BRAHIM Professeur Université Rennes 1 / directeur de thèse Table of contents Table of contents 1 Table of contents Introduction………………………………………………………………………………………7 Chapter 1 Microcrystalline silicon thin film transistors: Applications and technologies…..11 1.