Identification and Quantification of Volatile Organic Compounds in the Cape Town Brown Haze
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Achieving a Better Life for All Progress Between Census ‘96 and Census 2001 Statistics South Africa Private Bag X44 Pretoria 0001 South Africa
Statistics South Africa CENSUS 2001 Achieving a better life for all Progress between Census ‘96 and Census 2001 Statistics South Africa Private Bag X44 Pretoria 0001 South Africa 170 Andries Street Pretoria 0002 User information services: (012) 310 8600 Fax: (012) 310 8500 Main switchboard: (012) 310 8911 Website: www.statssa.gov.za Email: [email protected] Report no. 03-02-16 (2001) Statistics South Africa 2005 Pali Lehohla Statistician-General Published by Statistics South Africa, Private Bag X44, Pretoria 0001 © Statistics South Africa, 2005 Users may apply or process this data, provided Statistics South Africa (Stats SA) is acknowledged as the original source of the data; that it is specified that the application and/or analysis is the result of the user's independent processing of the data; and that neither the basic data nor any reprocessed version or application thereof may be sold or offered for sale in any form whatsoever without prior permission from Stats SA. Stats SA Library Cataloguing-in-Publication (CIP) Data Census 2001: Achieving a better life for all: Progress between Census ’96 and Census 2001 / Statistics South Africa. Pretoria: Statistics South Africa, 2005 215 p. (Report No. 03-02-16 (2001)) ISBN 0-621-34332-3 1. Population – South Africa 2. Statistics – South Africa 3. Census – South Africa 4. Households 5. Migration 6. Education 7. Disability I. Statistics South Africa (LCSH 16) A complete set of Stats SA publications is available at Stats SA Library and the following libraries: National Library of South Africa, -

Trans-Boundary Haze Pollution in Southeast Asia: Sustainability Through Plural Environmental Governance
sustainability Article Trans-Boundary Haze Pollution in Southeast Asia: Sustainability through Plural Environmental Governance Md Saidul Islam 1,*, Yap Hui Pei 2 and Shrutika Mangharam 1 1 Division of Sociology, Nanyang Technological University, 14 Nanyang Drive, Singapore 637332; [email protected] 2 Division of Psychology, Nanyang Technological University, 14 Nanyang Drive, Singapore 637332; [email protected] * Correspondence: [email protected]; Tel.: +65-6592-1519 Academic Editor: Marc A. Rosen Received: 29 February 2016; Accepted: 13 May 2016; Published: 21 May 2016 Abstract: Recurrent haze in Southeast Asian countries including Singapore is largely attributable to rampant forest fires in Indonesia due to, for example, extensive slash-and-burn (S & B) culture. Drawing on the “treadmill of production” and environmental governance approach, we examine causes and consequences of this culture. We found that, despite some perceived benefits, its environmental consequences include deforestation, soil erosion and degradation, global warming, threats to biodiversity, and trans-boundary haze pollution, while the societal consequences comprise regional tension, health risks, economic and productivity losses, as well as food insecurity. We propose sustainability through a plural coexistence framework of governance for targeting S & B that incorporates strategies of incentives, education and community resource management. Keywords: slash-and-burn; environmental governance; haze; Indonesia; plural coexistence; global warming; Singapore 1. Introduction The world’s rapidly growing population has been a long-standing cause of concern amongst both economists and environmentalists alike. There is an increasing demand for agricultural and urban spaces to sustain the ever-multiplying demographics. However, due to limited availability of space, the trend of clearing forests to make way for cultivable land has been gaining popularity [1]. -

An Initial Archaeological Assessment of Bloubergstrand
AN INITIAL ARCHAEOLOGICAL ASSESSMENT OF BLOUBERGSTRAND (FOR STRUCTURE PLAN PURPOSES ONLY) Prepared for Steyn Larsen and Partners December 1992 Prepared by Archaeology Contracts Office Department of Archaeology University of Cape Town Rondebosch 7700 Phone (021) 650 2357 Fax (021) 650 2352 1. INTRODUCTION The purpose of this report has been to identify areas of archaeological sensitivity in an area of Bloubergstrand as part of a local structure plan being compiled by Steyn, Larsen and Partners, Town and Regional Planners on behalf of the Western Cape Regional Services Council. The area of land which is examined is presented in Figure 1. Our brief specifically requested that we not undertake any detailed site identification. The conclusions reached are the result of an in loco inspection of the area and reference to observations compiled by members of the Archaeological Field Club1 during a visit to the area in 1978. While these records are useful they are not comprehensive and inaccuracies may be present. 2. BACKGROUND Human occupation of the coast and exploitation of marine resources was practised for many thousands of years before the colonisation of southern Africa by Europeans. This practise continued for some time after colonisation as well. Archaeological sites along the coast are often identified by the scatters of marine shells (middens) which accumulated at various places, sometimes in caves and rockshelters, but very often out in the open. Other food remains such as bones from a variety of faunas will often accompany the shells showing that the early inhabitants utilised the full range of resources of the coastal zone. -

Provincial Road Network Hibiscus Coast Local Municipality (KZ216)
O O O L L Etsheni P Sibukosethu Dunstan L L Kwafica 0 1 L 0 2 0 1 0 3 0 3 74 9 3 0 02 2 6 3 4 L1 .! 3 D923 0 Farrell 3 33 2 3 5 0 Icabhane 6 L0 4 D 5 2 L 3 7 8 3 O O 92 0 9 0 L Hospital 64 O 8 Empola P D D 1 5 5 18 33 L 951 9 L 9 D 0 0 D 23 D 1 OL 3 4 4 3 Mayiyana S 5 5 3 4 O 2 3 L 3 0 5 3 3 9 Gobhela 2 3 Dingezweni P 5 D 0 8 Rosettenville 4 L 1 8 O Khakhamela P 1 6 9 L 1 2 8 3 6 1 28 2 P 0 L 1 9 P L 2 1 6 O 1 0 8 1 1 - 8 D 1 KZN211L P6 19 8-2 P 1 P 3 0 3 3 3 9 3 -2 2 3 2 4 Kwazamokuhle HP - 2 L182 0 0 D Mvuthuluka S 9 1 N 0 L L 1 3 O 115 D -2 O D1113 N2 KZN212 D D D 9 1 1 1 Catalina Bay 1 Baphumlile CP 4 1 1 P2 1 7 7 9 8 !. D 6 5 10 Umswilili JP L 9 D 5 7 9 0 Sibongimfundo Velimemeze 2 4 3 6 Sojuba Mtumaseli S D 2 0 5 9 4 42 L 9 Mzingelwa SP 23 2 D 0 O O OL 1 O KZN213 L L 0 0 O L 2 3 2 L 1 2 Kwahlongwa P 7 3 Slavu LP 0 0 2 2 O 7 L02 7 3 32 R102 6 7 5 3 Buhlebethu S D45 7 P6 8-2 KZN214 Umzumbe JP St Conrad Incancala C Nkelamandla P 8 9 4 1 9 Maluxhakha P 9 D D KZN215 3 2 .! 50 - D2 Ngawa JS D 2 Hibberdene KwaManqguzuka 9 Woodgrange P N KZN216 !. -
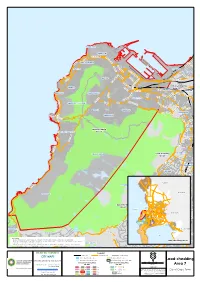
Load-Shedding Area 7
MOUILLE POINT GREEN POINT H N ELEN SUZMA H EL EN IN A SU M Z M A H N C THREE ANCHOR BAY E S A N E E I C B R TIO H A N S E M O L E M N E S SEA POINT R U S Z FORESHORE E M N T A N EL SO N PAARDEN EILAND M PA A A B N R N R D D S T I E E U H E LA N D R B H AN F C EE EIL A K ER T BO-KAAP R T D EN G ZO R G N G A KLERK E E N FW DE R IT R U A B S B TR A N N A D IA T ST S R I AN Load-shedding D D R FRESNAYE A H R EKKER L C Area 15 TR IN A OR G LBERT WOODSTOCK VO SIR LOWRY SALT RIVER O T R A N R LB BANTRY BAY A E TAMBOERSKLOOF E R A E T L V D N I R V R N I U M N CT LT AL A O R G E R A TA T E I E A S H E S ARL K S A R M E LIE DISTRICT SIX N IL F E V V O D I C O T L C N K A MIL PHILIP E O M L KG L SIGNAL HILL / LIONS HEAD P O SO R SAN I A A N M A ND G EL N ON A I ILT N N M TIO W STA O GARDENS VREDEHOEK R B PHILI P KGOSA OBSERVATORY NA F P O H CLIFTON O ORANJEZICHT IL L IP K K SANA R K LO GO E O SE F T W T L O E S L R ER S TL SET MOWBRAY ES D Load-shedding O RH CAMPS BAY / BAKOVEN Area 7 Y A ROSEBANK B L I S N WOO K P LSACK M A C S E D O RH A I R O T C I V RONDEBOSCH TABLE MOUNTAIN Load-shedding Area 5 KLIP PER N IO N S U D N A L RONDEBOSCH W E N D N U O R M G NEWLANDS IL L P M M A A A C R I Y N M L PA A R A P AD TE IS O E R P R I F 14 Swartland RIA O WYNBERG NU T C S I E V D CLAREMONT O H R D WOO BOW Drakenstein E OUDEKRAAL 14 D IN B U R G BISHOPSCOURT H RH T OD E ES N N A N Load-shedding 6 T KENILWORTH Area 11 Table Bay Atlantic 2 13 10 T Ocean R 1 O V 15 A Stellenbosch 7 9 T O 12 L 5 22 A WETTO W W N I 21 L 2S 3 A I A 11 M T E O R S L E N O D Hout Bay 16 4 O V 17 O A H 17 N I R N 17 A D 3 CONSTANTIA M E WYNBERG V R I S C LLANDUDNO T Theewaterskloof T E O 8 L Gordon's R CO L I N L A STA NT Bay I HOUT BAY IA H N ROCKLEY False E M H Bay P A L A I N MAI N IA Please Note: T IN N A G - Every effort has been made to ensure the accuracy of information in this map at the time of puMblication . -

Cape Town's Failure to Redistribute Land
CITY LEASES CAPE TOWN’S FAILURE TO REDISTRIBUTE LAND This report focuses on one particular problem - leased land It is clear that in order to meet these obligations and transform and narrow interpretations of legislation are used to block the owned by the City of Cape Town which should be prioritised for our cities and our society, dense affordable housing must be built disposal of land below market rate. Capacity in the City is limited redistribution but instead is used in an inefficient, exclusive and on well-located public land close to infrastructure, services, and or non-existent and planned projects take many years to move unsustainable manner. How is this possible? Who is managing our opportunities. from feasibility to bricks in the ground. land and what is blocking its release? How can we change this and what is possible if we do? Despite this, most of the remaining well-located public land No wonder, in Cape Town, so little affordable housing has been owned by the City, Province, and National Government in Cape built in well-located areas like the inner city and surrounds since Hundreds of thousands of families in Cape Town are struggling Town continues to be captured by a wealthy minority, lies empty, the end of apartheid. It is time to review how the City of Cape to access land and decent affordable housing. The Constitution is or is underused given its potential. Town manages our public land and stop the renewal of bad leases. clear that the right to housing must be realised and that land must be redistributed on an equitable basis. -

The History of Rondebosch Common a Plaque on a Stone at the South Cape Once Again
An Oa~i~ ~ A YQSt of flowQting or by Betty Dwight and Joanne Eastman, 1 ondebosch Common was declared a National Monument in 1961, thereby preserving, Runintentionally, a small piece of Cape Flats flora 'sand plain fynbos', of which so little remains, in the southern suburbs of Cape Town. Standing in the middle of the Common, surrounded by busy roads, one can still feel a sense of peace as the noise of the cars fade in the background. On closer inspection, with eyes turned to the ground, a wonderful miniature world of flowers, insects, birds and butterflies opens up. It is truly an oasis of wildness within the city. In January amongst the dry grass there are little patches of yellow Lobelia, Monopsis lutea. The African Monarch butterfly with its russet brown wings, tipped with black and white, flutters around the papery white Helichrysum flowers. Blue Aristea are flowering. This Aristea is taller with a strap-like stem compared to the Aristea africana seen in September. A few white Roella prostrata straggle along the ground. The Psoralea pinnata shrubs with their pale mauve flowers appear bravely in the dry season. Even in hot February there is something to find. The Struthiola shrublets are covered in small creamy, sometimes pale pink flowers. The restios stand out amongst the yellow grass with their green stems and dark brown flower heads. There are also many interesting seed-pods beginning to form. Grasshoppers, dragonflies and the Citrus Swallowtail butterflies are evident. In March the large black ants are on the move, very busy carrying seeds to their nests. -

Greater Cape Metro Regional Spatial Implementation Framework Final Report July 2019
Greater Cape Metro Regional Spatial Implementation Framework Final Report July 2019 FOREWORD The Western Cape Government will advance the spatial transformation of our region competitive advantages (essentially tourism, food and calls on us all to give effect to a towards greater resilience and spatial justice. beverages, and education) while anticipating impacts of technological innovation, climate change and spatial transformation agenda The Department was challenged to explore the urbanization. Time will reveal the extent to which the which brings us closer to the linkages between planning and implementation dynamic milieu of demographic change, IT advances, imperatives of growing and and to develop a Greater Cape Metropolitan the possibility of autonomous electric vehicles and sharing economic opportunities Regional Implementation Framework (GCM RSIF) climate change (to name a few) will affect urban and wherever we are able to impact rather than “just another plan” which will gravitate to regional morphology. The dynamic environment we upon levers of change. Against the bookshelf and not act as a real catalyst for the find ourselves in is underscored by numerous potential the background of changed implementation of a regional logic. planning legislation, and greater unanticipated impacts. Even as I pen this preface, clarity regarding the mandates of agencies of This GCM RSIF is the first regional plan to be approved there are significant issues just beyond the horizon governance operating at different scales, the PSDF in terms of the Western Cape Land Use Planning Act, for this Province which include scientific advances in 2014 remained a consistent guide and mainspring, 2014. As such it offered the drafters an opportunity (a AI, alternative fuel types for transportation (electric prompting us to give urgent attention to planning in kind of “laboratory”) to test processes and procedures vehicles and hydrogen power) and the possibility the Greater Cape Metropolitan Region as one of three in the legislation. -

Acute Health Impacts of the Southeast Asian Transboundary Haze Problem—A Review
International Journal of Environmental Research and Public Health Review Acute Health Impacts of the Southeast Asian Transboundary Haze Problem—A Review Kang Hao Cheong 1,* , Nicholas Jinghao Ngiam 2 , Geoffrey G. Morgan 3, Pin Pin Pek 4,5, Benjamin Yong-Qiang Tan 2, Joel Weijia Lai 1, Jin Ming Koh 1, Marcus Eng Hock Ong 4,5 and Andrew Fu Wah Ho 6,7,8 1 Science and Math Cluster, Singapore University of Technology and Design, Singapore 487372, Singapore 2 Division of Neurology, Department of Medicine, National University Health System, Singapore 119074, Singapore 3 School of Public Health, The University of Sydney, Sydney, NSW 2006, Australia 4 Department of Emergency Medicine, Singapore General Hospital, Singapore 169608, Singapore 5 Health Services & Systems Research, Duke-NUS Medical School, Singapore 169857, Singapore 6 SingHealth Duke-NUS Emergency Medicine Academic Clinical Programme, Singapore 169857, Singapore 7 National Heart Research Institute Singapore, National Heart Centre, Singapore 169609, Singapore 8 Cardiovascular & Metabolic Disorders Programme, Duke-NUS Medical School, Singapore 169857, Singapore * Correspondence: [email protected] Received: 20 August 2019; Accepted: 29 August 2019; Published: 6 September 2019 Abstract: Air pollution has emerged as one of the world’s largest environmental health threats, with various studies demonstrating associations between exposure to air pollution and respiratory and cardiovascular diseases. Regional air quality in Southeast Asia has been seasonally affected by the transboundary haze problem, which has often been the result of forest fires from “slash-and-burn” farming methods. In light of growing public health concerns, recent studies have begun to examine the health effects of this seasonal haze problem in Southeast Asia. -

Activism in Manenberg, 1980 to 2010
Then and Now: Activism in Manenberg, 1980 to 2010 Julian A Jacobs (8805469) University of the Western Cape Supervisor: Prof Uma Dhupelia-Mesthrie Masters Research Essay in partial fulfillment of Masters of Arts Degree in History November 2010 DECLARATION I declare that „Then and Now: Activism in Manenberg, 1980 to 2010‟ is my own work and that all the sources I have used or quoted have been indicated and acknowledged by means of complete references. …………………………………… Julian Anthony Jacobs i ABSTRACT This is a study of activists from Manenberg, a township on the Cape Flats, Cape Town, South Africa and how they went about bringing change. It seeks to answer the question, how has activism changed in post-apartheid Manenberg as compared to the 1980s? The study analysed the politics of resistance in Manenberg placing it within the over arching mass defiance campaign in Greater Cape Town at the time and comparing the strategies used to mobilize residents in Manenberg in the 1980s to strategies used in the period of the 2000s. The thesis also focused on several key figures in Manenberg with a view to understanding what local conditions inspired them to activism. The use of biographies brought about a synoptic view into activists lives, their living conditions, their experiences of the apartheid regime, their brutal experience of apartheid and their resistance and strength against a system that was prepared to keep people on the outside. This study found that local living conditions motivated activism and became grounds for mobilising residents to make Manenberg a site of resistance. It was easy to mobilise residents on issues around rent increases, lack of resources, infrastructure and proper housing. -

The Criteria Pollutants & a Closer Look at Ozone
Module 1: Activity 4 The Criteria Pollutants & a Closer Look at Ozone SUMMARY Students will be introduced to the criteria pollutants and will work in groups to make cartoon booklets demonstrating the formation of ground-level ozone and the destruction of stratospheric ozone. ESSENTIAL QUESTIONS • What are criteria pollutants and why are they of concern? • What’s the difference between stratospheric ozone and ground-level ozone? • Why do we sometimes have too much ozone in the troposphere and not enough in the stratosphere? • How do these problems affect human health? TIME NEEDED Highly variable. It’s possible to complete this activity in one block period if you just use the background information provided and have students create a comic strip individually. However if you want to give students more time to produce creative products, allow two full days to work on the project in groups, and 30 minutes to present projects in class. 2012 North Carolina ESSENTIAL STANDARDS FOR EARTH/ENVIRONMENTAL SCIENCE • EEn.2.5.1 Summarize the structure and composition of our atmosphere. • EEn.2.5.5 Explain how human activities affect air quality. • EEn.2.7.3 Explain how human activities impact the biosphere. A product of the NC Air Awareness Program 2014. Available for re-distribution with permission. [email protected] www.itsourair.org The Criteria Pollutants & Module 1: a Closer Look at Ozone Activity 4 (usually over 75° F). NOX comes from burning fuels. VOCs are MAKING chemicals containing carbon that evaporate easily – which usually means they are smelly. Sources of VOCs include CONNECTIONS cleaning chemicals, solvents, paints, unburned gasoline, hog In this activity, students will be learning about ozone waste lagoons, and natural sources such as trees. -

The Chesterfield Brand New Development, Rondebosch
THE CHESTERFIELD BRAND NEW DEVELOPMENT, RONDEBOSCH THE NOVA GROUP PROPERTY DEVELOPMENT & CONSTRUCTION HEAD OFFICE 021 433 2580 SEA POINT 021 434 1223 dogongroup.com A visionary company with decades of experience COMPANY PARTICULARS Established by our CEO, Denise Dogon in 2002, Dogon Group Properties has proven to be a true real estate success story. The Dogon name has become synonymous with the proficient and effective marketing and selling of premium grade properties in Cape Town and particularly the sought-after Atlantic Seaboard. Dogon Group currently have offices in high profile positions in the City Bowl, Sea Point, Southern Suburbs and Gauteng. Dogon Group Properties prides itself on its unique and focused approach to marketing and sales, providing a comprehensive and tailored solution to ensure that sales occur at the optimum price within a compressed space of time. The company utilizes an evolved and distinctive sales force of highly adept and skilled sales agents who are selected for their extensive experience, professionalism and successful track records. Headed by CEO, Denise Dogon (Head of Marketing) and Managing Director Alexa Horne, Dogon Group has a dedicated in- house marketing department ensuring that focused and specialized marketing strategies are implemented. The powerful proprietary Dogon Group database combined with its eye-catching and prominent advertising, both in print and digital media, together with our visible network of sales offices support our strong performance on the Western Cape’s Atlantic Seaboard,