Discordance Between Genomic Divergence and Phenotypic Variation in a T Rapidly Evolving Avian Genus (Motacilla) ⁎ Rebecca B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Birds (Aves) of Oromia, Ethiopia – an Annotated Checklist
European Journal of Taxonomy 306: 1–69 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.306 www.europeanjournaloftaxonomy.eu 2017 · Gedeon K. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:A32EAE51-9051-458A-81DD-8EA921901CDC The birds (Aves) of Oromia, Ethiopia – an annotated checklist Kai GEDEON 1,*, Chemere ZEWDIE 2 & Till TÖPFER 3 1 Saxon Ornithologists’ Society, P.O. Box 1129, 09331 Hohenstein-Ernstthal, Germany. 2 Oromia Forest and Wildlife Enterprise, P.O. Box 1075, Debre Zeit, Ethiopia. 3 Zoological Research Museum Alexander Koenig, Centre for Taxonomy and Evolutionary Research, Adenauerallee 160, 53113 Bonn, Germany. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 1 urn:lsid:zoobank.org:author:F46B3F50-41E2-4629-9951-778F69A5BBA2 2 urn:lsid:zoobank.org:author:F59FEDB3-627A-4D52-A6CB-4F26846C0FC5 3 urn:lsid:zoobank.org:author:A87BE9B4-8FC6-4E11-8DB4-BDBB3CFBBEAA Abstract. Oromia is the largest National Regional State of Ethiopia. Here we present the first comprehensive checklist of its birds. A total of 804 bird species has been recorded, 601 of them confirmed (443) or assumed (158) to be breeding birds. At least 561 are all-year residents (and 31 more potentially so), at least 73 are Afrotropical migrants and visitors (and 44 more potentially so), and 184 are Palaearctic migrants and visitors (and eight more potentially so). Three species are endemic to Oromia, 18 to Ethiopia and 43 to the Horn of Africa. 170 Oromia bird species are biome restricted: 57 to the Afrotropical Highlands biome, 95 to the Somali-Masai biome, and 18 to the Sudan-Guinea Savanna biome. -

South Africa: Magoebaskloof and Kruger National Park Custom Tour Trip Report
SOUTH AFRICA: MAGOEBASKLOOF AND KRUGER NATIONAL PARK CUSTOM TOUR TRIP REPORT 24 February – 2 March 2019 By Jason Boyce This Verreaux’s Eagle-Owl showed nicely one late afternoon, puffing up his throat and neck when calling www.birdingecotours.com [email protected] 2 | TRIP REPORT South Africa: Magoebaskloof and Kruger National Park February 2019 Overview It’s common knowledge that South Africa has very much to offer as a birding destination, and the memory of this trip echoes those sentiments. With an itinerary set in one of South Africa’s premier birding provinces, the Limpopo Province, we were getting ready for a birding extravaganza. The forests of Magoebaskloof would be our first stop, spending a day and a half in the area and targeting forest special after forest special as well as tricky range-restricted species such as Short-clawed Lark and Gurney’s Sugarbird. Afterwards we would descend the eastern escarpment and head into Kruger National Park, where we would make our way to the northern sections. These included Punda Maria, Pafuri, and the Makuleke Concession – a mouthwatering birding itinerary that was sure to deliver. A pair of Woodland Kingfishers in the fever tree forest along the Limpopo River Detailed Report Day 1, 24th February 2019 – Transfer to Magoebaskloof We set out from Johannesburg after breakfast on a clear Sunday morning. The drive to Polokwane took us just over three hours. A number of birds along the way started our trip list; these included Hadada Ibis, Yellow-billed Kite, Southern Black Flycatcher, Village Weaver, and a few brilliant European Bee-eaters. -

29Th 2019-Uganda
AVIAN SAFARIS 23 DAY UGANDA BIRDING AND NATURE TOUR ITINERARY Date: July 7 July 29, 2019 Tour Leader: Crammy Wanyama Trip Report and all photos by Crammy Wanyama Black-headed Gonolek a member of the Bush-shrikes family Day 1 – July 7, 2019: Beginning of the tour This tour had uneven arrivals. Two members arrived two days earlier and the six that came in on the night before July 7th, stayed longer; therefore, we had a pre and post- tour to Mabira Forest. For today, we all teamed up and had lunch at our accommodation for the next two nights. This facility has some of the most beautiful gardens around Entebbe; we decided to spend the rest of the afternoon here watching all the birds you would not expect to find around a city garden. Some fascinating ones like the Black-headed Gonolek nested in the garden, White-browed Robin-Chat too did. The trees that surrounded us offered excellent patching spots for the African Hobby. Here we had a Falco patching out in the open for over forty minutes! Superb looks at a Red-chested and Scarlet-chested Sunbirds. The gardens' birdbath attracted African Thrush that reminded the American birders of their American Robin, Yellow- throated Greenbul. Still looking in the trees, we were able to see African Grey Woodpeckers, both Meyer's and Grey Parrot, a pair of Red-headed Lovebirds. While walking around the facility, we got good looks at a flying Shikra and spent ample time with Ross's Turaco that flew back and forth. We had a very lovely Yellow-fronted Tinkerbird on the power lines, Green-backed Camaroptera, a very well sunlit Avian Safaris: Email: [email protected] Website: http://www.aviansafaris.com AVIAN SAFARIS Spectacled Weaver, was added on the Village and Baglafecht Weavers that we had seen earlier and many more. -

ETHIOPIA: Birding the Roof of Africa; with Southern Extension a Tropical Birding Set Departure
ETHIOPIA: Birding the Roof of Africa; with Southern Extension A Tropical Birding Set Departure February 7 – March 1, 2010 Guide: Ken Behrens All photos taken by Ken Behrens during this trip ORIENTATION I have chosen to use a different format for this trip report. First, comes a general introduction to Ethiopia. The text of this section is largely drawn from the recently published Birding Ethiopia, authored by Keith Barnes, Christian, Boix and I. For more information on the book, check out http://www.lynxeds.com/product/birding-ethiopia. After the country introduction comes a summary of the highlights of this tour. Next comes a day-by-day itinerary. Finally, there is an annotated bird list and a mammal list. ETHIOPIA INTRODUCTION Many people imagine Ethiopia as a flat, famine- ridden desert, but this is far from the case. Ethiopia is remarkably diverse, and unexpectedly lush. This is the ʻroof of Africaʼ, holding the continentʼs largest and most contiguous mountain ranges, and some of its tallest peaks. Cleaving the mountains is the Great Rift Valley, which is dotted with beautiful lakes. Towards the borders of the country lie stretches of dry scrub that are more like the desert most people imagine. But even in this arid savanna, diversity is high, and the desert explodes into verdure during the rainy season. The diversity of Ethiopiaʼs landscapes supports a parallel diversity of birds and other wildlife, and although birds are the focus of our tour, there is much more to the country. Ethiopia is the only country in Africa that was never systematically colonized, and Rueppell’s Robin-Chat, a bird of the Ethiopian mountains. -

Sierra Leone
SIERRA LEONE 9 - 24 FEBRUARY 2008 TOUR REPORT LEADER: NIK BORROW Our first exploratory tour to Sierra Leone was pretty tough going at times but certainly pulled a few goodies out of the bag! A respectable total of 305 species were recorded of which all but 12 were seen. The notable major highlights had to be the wonderful views of the amazing Yellow-headed Picathartes preening and posing at their nest site before going to roost, the restricted range Turati’s Boubou and no less than four stunning Gola Malimbes for everyone! Singing Brown Nightjars were discovered, sublime Egyptian Plovers enjoyed, colourful Buff-throated Sunbirds enthralled and secretive Capuchin Babblers were tracked down. Mammals were sparse but we had great looks at the beautiful Diana Monkey and Olive Colobus and we even almost saw a Pygmy Hippo that crashed away from us through the undergrowth! Other specialties included Red-chested Goshawk, Latham’s Forest Francolin, Black-shouldered and Standard-winged Nightjars, Blue-headed Bee-eater, Brown- cheeked and Yellow-casqued Hornbills, Hairy-breasted Barbet, Spotted Honeyguide, Little Green, Melancholy and Fire-bellied Woodpeckers, Fanti Saw-wing, Preuss’s Cliff Swallow, Pied-winged Swallow, Green-tailed and Grey-headed Bristlebills, Western Bearded Greenbul, Yellow-bearded Greenbul, Western Forest Robin, White-tailed Alethe, Finsch’s Flycatcher Thrush, Forest Scrub Robin, Sharpe’s Apalis, Kemp’s Longbill, Olivaceous and Ussher’s Flycatchers, Red-cheeked Wattle-eye, Rufous-winged and Puvel’s Illadopsis, Red-billed Helmet-shrike, Copper-tailed Glossy and Emerald Starlings, Maxwell’s Black Weaver, Red-vented Malimbe, Yellow-winged Pytilia and Dybowski’s Twinspot. -

Status and Distribution of Faunal Diversity in Kafa Afromontane Coffee Forest
Status and Distribution of Faunal Diversity in Kafa Afromontane Coffee Forest Leykun Abunie Berhan Submitted to PPP Project July 2008 Addis Ababa Contents Executive Summary .....................................................................................................................4 Introduction..................................................................................................................................6 Literature Review Related to Faunal Diversity and Management...............................................8 Macro Policies and Priorities......................................................................................................8 Environmental Protection Policy.................................................................................................8 Wildlife Development / Management Policy................................................................................9 Analysis of Wildlife Sector in Ethiopia ......................................................................................10 Physical and Ecological Description of the Study Area ............................................................14 Objective of the Present Study...................................................................................................16 Methodology ..............................................................................................................................17 General Approach......................................................................................................................17 -

W. TOMLINSON, Bird Notes from the NF District
JAN. 1950 W. TOMLINSON, Bird Notes from the N.F. District 225 BIRD NOTES CHIEFLY FROM THE NORTHERN FRONTIER DISTRICT OF KENYA PART II by W. Tomlinson. ALAUDIDAE Mirafra albicauda Reichenow. White-tailed Lark. Thika, April. Mirafra hypermetra hypermetra (Reichenow) Red-winged Bush-lark. Angata Kasut; Merille; Thika. In the Kasut to the south-west of Marsabit Mountain this large lark was fairly common,haunting a patch of desert where there was some bush and even grass following rain. Runs well in quick spurts; but, pursued, usually takes wing. The flight is strong, although it seldom goes far before ducking down again, usually perching on tops of bushes. In the heat of the day seen sheltering under bush. Has a clear and loud, two• Il)ted call and a very pretty song of four or fivenotes. The cinnamon-rufous of its plum• age shows in flight but is invisible in the bird when at rest. At Merille, the Kasut and at Th;ka, where this bird occurred, the ground was the same russet colour as the bird's plumage. Mirafra africana dohertyi Hartert Kikuyu Red-naped Lark. Thika; Nanyuki. Mirafra fischeri fischeri (Reichenow) Flappet Lark. Thika. Mirafra africanoides intercedens Reichenow. Masai Fawn-coloured Lark. Merille; North Horr. A " flappet-Iark," fairly common at Merille in January, was I think this. At the time a small cricket was in thousands in patches of open country, followed up by Larks and Wattled Starlings. Mirafra poecilosterna poecilosterna (Reichenow). Pink-breasted Singing Lark. Merille. A bird frequently flushed from the ground, which flew to bushes and low trees, was I think this. -

Environmental Impact Assessment Report for Pungwe B Hydroelectric 2013 Electric Power Scheme
Environmental Impact Assessment Report for Pungwe B Hydroelectric 2013 Electric Power Scheme Proponent Nyangani Renewable Energy Date January 2013 Document Title ENVIRONMENTAL IMPACT ASSESSMENT Report for the Pungwe B hydroelectric power scheme on the Pungwe River in the Honde Valley, north eastern Zimbabwe Black Crystal Consulting Private Limited EMA Reg. No. 00004/2011 1 Fairbairn Drive Mt Pleasant Harare Box 9111 Harare Phone: (04) 33 43 61 / 2912645 Black Crystal Consulting Private Limited EMA Reg. No. 0000071/2013 1 Fairbairn Drive Mt Pleasant Harare Box 9111 Harare Phone: (04) 334 361/ 307 458/ 2912645 Fax : (04) 307466 Mobile : +263 779 394 179 E-mail: [email protected] www.blackcrystal.co.zw Environmental Impact Assessment Report for Pungwe B Hydroelectric 2013 Electric Power Scheme Copyright © 2013 by Black Crystal Consulting (Private) Limited This report is the sole property of Nyangani Renewable Energy and Black Crystal Consulting. All rights reserved. No part of this report may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior written permission of the proponent and the consultants, nor be otherwise circulated in any form of binding or cover other than that in which it is produced here. Final Report Page ii Environmental Impact Assessment Report for Pungwe B Hydroelectric 2013 Electric Power Scheme CONSULTANT DETAILS: Black Crystal Consulting Private Limited EMA Reg. No. 000071/2012 Harare Office: No. 1 Fairbairn Drive Mount Pleasant HARARE. P O Box 9111, Harare. E-mail: [email protected] www.blackcrystal.co.zw Phone: (04) 334 361/ 307 458/ 2912645 Fax : (04) 307466 Mobile : +263 779 394 179 Bulawayo Office: P O Box FM 493, Famona Bulawayo Mobile +263 772 126 963 Final Report Page iii Environmental Impact Assessment Report for Pungwe B Hydroelectric 2013 Electric Power Scheme CONSULTANT TEAM MEMBERS: ECOLOGIST: Ms S.L. -

Havens of Biodiversity, and Places That Allow People to Connect with Natural Habitats and Ecosystems, Will Become Increasingly More Valuable for Future Generations
Supplement to Veld & Flora, Vol. 93(4) December 2007 1 booklet3_FINAL_for print.indd 1 2007/11/02 10:50:33 AM FOREWORD The Botanical Society of South Africa (BotSoc) has been a partner and supporter of the South African National Biodiversity Institute (SANBI) and its forerunners for over 90 years. This supplement to Veld & Flora focuses on other “biodiversity” (birds, mammals, insects, etc.) rather than just our core interest, which is “plant diversity”. It is an example of BotSoc embracing the change which Dr Bruce McKenzie has come about since SANBI replaced its predecessor Executive Director, BotSoc the National Botanical Institute (NBI) and also supports one of the principles contained in BotSoc’s Centenary Charter (see Veld & Flora, March 2006) which outlines our commitment to supporting SANBI and its mandate. In this regard the BotSoc warmly welcomes the first CEO of SANBI, Dr Tanya Abrahamse, and looks forward to working with her and her team in tackling new challenges, some of which she has spelt out in her foreword to the supplement. Dr Bruce McKenzie EXECUTIVE DIRECTOR, BotSoc CONTENTS 2 Animals form an integral part of South Africa’s National Botanical Gardens 3 Free State NBG, Bloemfontein 4 Harold Porter NBG, Betty’s Bay 6 Karoo Desert NBG, Worcester 7 Kirstenbosch NBG, Cape Town KwaZulu-Natal NBG, Pietermaritzburg Compiled by: 11 Christopher K. Willis & 13 Lowveld NBG, Nelspruit Augustine T. Morkel 16 Nieuwoudtville NBG Published by: The Botanical Society of South Africa 18 Pretoria NBG and the South African National 21 -
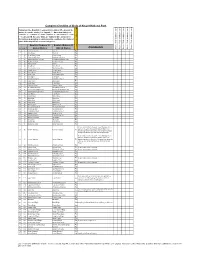
Kruger Comprehensive
Complete Checklist of birds of Kruger National Park Status key: R = Resident; S = present in summer; W = present in winter; E = erratic visitor; V = Vagrant; ? - Uncertain status; n = nomadic; c = common; f = fairly common; u = uncommon; r = rare; l = localised. NB. Because birds are highly mobile and prone to fluctuations depending on environmental conditions, the status of some birds may fall into several categories English (Roberts 7) English (Roberts 6) Comments Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps # Rob # Global Names Old SA Names Rough Status of Bird in KNP 1 1 Common Ostrich Ostrich Ru 2 8 Little Grebe Dabchick Ru 3 49 Great White Pelican White Pelican Eu 4 50 Pinkbacked Pelican Pinkbacked Pelican Er 5 55 Whitebreasted Cormorant Whitebreasted Cormorant Ru 6 58 Reed Cormorant Reed Cormorant Rc 7 60 African Darter Darter Rc 8 62 Grey Heron Grey Heron Rc 9 63 Blackheaded Heron Blackheaded Heron Ru 10 64 Goliath Heron Goliath Heron Rf 11 65 Purple Heron Purple Heron Ru 12 66 Great Egret Great White Egret Rc 13 67 Little Egret Little Egret Rf 14 68 Yellowbilled Egret Yellowbilled Egret Er 15 69 Black Heron Black Egret Er 16 71 Cattle Egret Cattle Egret Ru 17 72 Squacco Heron Squacco Heron Ru 18 74 Greenbacked Heron Greenbacked Heron Rc 19 76 Blackcrowned Night-Heron Blackcrowned Night Heron Ru 20 77 Whitebacked Night-Heron Whitebacked Night Heron Ru 21 78 Little Bittern Little Bittern Eu 22 79 Dwarf Bittern Dwarf Bittern Sr 23 81 Hamerkop -

The 55 Species of Larger Mammal Known to Be Present in The
Birds of Lolldaiga Hills Ranch¹ Order and scientific name² Common name² Threat3 Comments Struthionidae Ostrich Struthio camelus Common ostrich LC Both S. c. camelus (LC) and S. c. molybdophanes (Somali ostrich) (VU) present. These considered species by some authorities. Numididae Guineafowl Numida meleagris Helmeted guineafowl LC Acryllium vulturinum Vulturine guineafowl LC Phasianidae Stone partridge, francolins, spurfowl, quails Ptilopachus petrosus Stone partridge LC Francolinus shelleyi Shelley’s francolin LC Francolinus sephaena Crested francolin LC Francolinus squamatus Scaly francolin LC Francolinus hildebrandti Hildebrandt’s francolin LC Francolinus leucoscepus Yellow-necked spurfowl LC Coturnix coturnix Common quail LC Coturnix delegorguei Harlequin quail LC Anatidae Ducks, geese Dendrocygna viduata White-faced whistling duck LC Sarkidiornis melanotos Knob-billed duck LC Alopochen aegyptiaca Egyptian goose LC Anas strepera Gadwall LC Anas sparsa African black duck LC Anas undulata Yellow-billed duck LC 1 Order and scientific name² Common name² Threat3 Comments Anas clypeata Northern shoveler LC Anas erythrorhyncha Red-billed teal LC Anas acuta Northern pintail LC Anas querquedula Garganey LC Anas crecca Eurasian teal LC Anas hottentota Hottentot teal LC Netta erythrophthalma Southern pochard LC Oxyura maccoa Maccoa duck NT Podicipedidae Grebes Tachybaptus ruficollis20 Little grebe LC Ciconiidae Storks Mycteria ibis Yellow-billed stork LC Anastomus lamelligerus African open-billed stork LC Ciconia nigra Black stork LC Ciconia abdimii -

Assessment of the Primates, Large Mammals and Birds of the Mathews Range Forest Reserve, Central Kenya
Assessment of the Primates, Large Mammals and Birds of the Mathews Range Forest Reserve, Central Kenya Yvonne A. de Jong Thomas M. Butynski Eastern Africa Primate Diversity and Conservation Program Report to The Nature Conservancy, Washington D.C. August 2010 Mathews Range Assessment, August 2010 Assessment of the Primates, Large Mammals and Birds of the Mathews Range Forest Reserve, Central Kenya Yvonne A. de Jong Eastern Africa Primate Diversity and Conservation Program P.O. Box 149 10400 Nanyuki, Kenya [email protected] Thomas M. Butynski Zoological Society of London King Khalid Wildlife Research Center P.O. Box 61681 Riyadh 11575, Saudi Arabia [email protected] Report to The Nature Conservancy, Washington D.C. August 2010 Yvonne A. de Jong & Thomas M. Butynski Eastern Africa Primate Diversity and Conservation Program Website: www.wildsolutions.nl Recommended citation: De Jong, Y.A. & Butynski, T.M. 2010. Assessment of the primates, large mammals and birds of the Mathews Range Forest Reserve, central Kenya. Unpublished report to The Nature Conservancy, Washington D.C. Cover photo: Left: Elder Lpaasion Lesipih and scout Sinyah Lesowapir. Upper right: pearl- spotted owlet Glaucidium perlatum. Lower right: bushbuck Tragelaphus scriptus. All photographs taken in the Mathews Range by Yvonne A. de Jong and Thomas M. Butynski. 2 Y.A. de Jong & T.M. Butynski CONTENTS Summary 4 Introduction 6 Methods 7 Research Area 8 Results, Conclusions and Discussion 10 I. Primates 10 Small-eared greater galago Otolemur garnettii 12 Somali galago Galago gallarum 14 Mt. Uarges Guereza Colobus guereza percivali 17 De Brazza’s monkey Cercopithecus neglectus 20 Hilgert’s vervet monkey Chlorocebus pygerythrus hilgerti 22 Olive baboon Papio anubis 24 Conclusion and Discussion 27 Primate Conservation in the Mathews Range 28 II.