PRAC Recommendations on Signals Adopted at the 13-16 January 2020 PRAC En
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
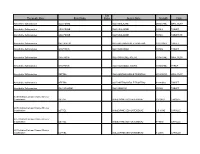
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

PEMD-91-12BR Off-Label Drugs: Initial Results of a National Survey
11 1; -- __...._-----. ^.-- ______ -..._._ _.__ - _........ - t Ji Jo United States General Accounting Office Washington, D.C. 20648 Program Evaluation and Methodology Division B-242851 February 25,199l The Honorable Edward M. Kennedy Chairman, Committee on Labor and Human Resources United States Senate Dear Mr. Chairman: In September 1989, you asked us to conduct a study on reimbursement denials by health insurers for off-label drug use. As you know, the Food and Drug Administration designates the specific clinical indications for which a drug has been proven effective on a label insert for each approved drug, “Off-label” drug use occurs when physicians prescribe a drug for clinical indications other than those listed on the label. In response to your request, we surveyed a nationally representative sample of oncologists to determine: . the prevalence of off-label use of anticancer drugs by oncologists and how use varies by clinical, demographic, and geographic factors; l the extent to which third-party payers (for example, Medicare intermediaries, private health insurers) are denying payment for such use; and l whether the policies of third-party payers are influencing the treatment of cancer patients. We randomly selected 1,470 members of the American Society of Clinical Oncologists and sent them our survey in March 1990. The sam- pling was structured to ensure that our results would be generalizable both to the nation and to the 11 states with the largest number of oncologists. Our response rate was 56 percent, and a comparison of respondents to nonrespondents shows no noteworthy differences between the two groups. -

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Information for the User ZYTIGA 500 Mg Film-Coated Tablets Abiraterone
Package leaflet: Information for the user ZYTIGA 500 mg film-coated tablets abiraterone acetate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What ZYTIGA is and what it is used for 2. What you need to know before you take ZYTIGA 3. How to take ZYTIGA 4. Possible side effects 5. How to store ZYTIGA 6. Contents of the pack and other information 1. What ZYTIGA is and what it is used for ZYTIGA contains a medicine called abiraterone acetate. It is used to treat prostate cancer in adult men that has spread to other parts of the body. ZYTIGA stops your body from making testosterone; this can slow the growth of prostate cancer. When ZYTIGA is prescribed for the early stage of disease where it is still responding to hormone therapy, it is used with a treatment that lowers testosterone (androgen deprivation therapy ). When you take this medicine your doctor will also prescribe another medicine called prednisone or prednisolone. This is to lower your chances of getting high blood pressure, having too much water in your body (fluid retention), or having reduced levels of a chemical known as potassium in your blood. -

Abiraterone Acetate for Chemotherapy-Naive
Fan et al. BMC Urology (2018) 18:110 https://doi.org/10.1186/s12894-018-0416-6 RESEARCHARTICLE Open Access Abiraterone acetate for chemotherapy- naive metastatic castration-resistant prostate cancer: a single-centre prospective study of efficacy, safety, and prognostic factors Liancheng Fan†, Baijun Dong†, Chenfei Chi†, Yanqing Wang†, Yiming Gong†, Jianjun Sha, Jiahua Pan, Xun Shangguan, Yiran Huang, Lixin Zhou* and Wei Xue* Abstract Background: To evaluate the efficacy and safety of abiraterone acetate (AA) plus prednisone compared with prednisone alone in Asian patients with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC), and to identify predictive factors. Methods: We reviewed the medical records of 60 patients with chemotherapy-naive mCRPC at Renji Hospital who were treated with AA plus prednisone (n = 43) or prednisone alone (n = 17). All patients were assessed for prostate- specific antigen (PSA) response, PSA progression-free survival (PSA PFS), radiographic progression-free survival (rPFS), and overall survival (OS). The ability of several parameters to predict PSA PFS, rPFS, and OS was studied. Results: The median follow-up time was 14.0 months (range 7.0–18.5 months), at which time 19 death events had been reported: 11 in the AA + prednisone group and 8 in the prednisone group. The AA + prednisone group had significantly longer median PSA PFS (10.3 vs 3.0 months, P < 0.001), rPFS (13.9 vs 3.9 months, P < 0.001), and OS (23. 3 vs 17.5 months, P = 0.016) than the prednisone-alone group. The most frequently reported grade 3 or 4 adverse event in both the AA + prednisone and prednisone-alone groups was elevated alanine aminotransferase level in 5 of 43 patients (11.6%) and 2 of 17 patients (11.8%), respectively. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

YONSA (Abiraterone Acetate) Tablets May Have Different Dosing and Food Effects Than Other Abiraterone Acetate Products
HIGHLIGHTS OF PRESCRIBING INFORMATION hypokalemia before treatment. Monitor blood pressure, serum These highlights do not include all the information needed to use YONSA potassium and symptoms of fluid retention at least monthly. (5.1) safely and effectively. See full prescribing information for YONSA. • Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids YONSA® (abiraterone acetate) tablets, for oral use may be indicated before, during and after stressful situations. (5.2) Initial U.S. Approval: 2011 • Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue YONSA dosing as recommended. ----------------------------INDICATIONS AND USAGE-------------------------- YONSA is a CYP17 inhibitor indicated in combination with (5.3) methylprednisolone for the treatment of patients with metastatic castration- resistant prostate cancer (CRPC). (1) ------------------------------ADVERSE REACTIONS------------------------------ The most common adverse reactions (≥ 10%) are fatigue, joint swelling or ----------------------DOSAGE AND ADMINISTRATION---------------------- discomfort, edema, hot flush, diarrhea, vomiting, cough, hypertension, To avoid medication errors and overdose, be aware that YONSA tablets may dyspnea, urinary tract infection and contusion. have different dosing and food effects than other abiraterone acetate products. Recommended dose: YONSA 500 mg (four 125 mg tablets) administered The most common laboratory -

Ep 3027638 B1
(19) TZZ¥Z ¥_T (11) EP 3 027 638 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07J 31/00 (2006.01) C07J 43/00 (2006.01) 27.12.2017 Bulletin 2017/52 (86) International application number: (21) Application number: 13771622.1 PCT/IB2013/056206 (22) Date of filing: 29.07.2013 (87) International publication number: WO 2015/015246 (05.02.2015 Gazette 2015/05) (54) PROCESS FOR THE PREPARATION OF ABIRATERONE AND ABIRATERONE ACETATE VERFAHREN ZUR HERSTELLUNG VON ABIRATERON ODER ABIRATERONACETAT PROCÉDÉ DE PRÉPARATION D’ABIRATÉRONE ET D’ACÉTATE D’ABIRATÉRONE (84) Designated Contracting States: (56) References cited: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB WO-A1-93/20097 WO-A1-2006/021777 GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO WO-A2-2014/207567 PL PT RO RS SE SI SK SM TR • SUN Q ET AL: "Pd(PPh3)4/AgOAc-catalyzed (43) Date of publication of application: coupling of 17-steroidal triflates and alkynes: 08.06.2016 Bulletin 2016/23 Highly efficient synthesis of D-ring unsaturated 17-alkynylsteroids", STEROIDS, ELSEVIER (73) Proprietor: Industriale Chimica S.R.L. SCIENCE PUBLISHERS, NEW YORK, NY, US, vol. 20145 Milano (IT) 75, no. 12, 1 December 2010 (2010-12-01), pages 936-943, XP027221112, ISSN: 0039-128X (72) Inventors: [retrieved on 2010-06-01] • LENNA, Roberto I-20010 S. Giorgio Su Legnano (IT) Remarks: • DI BRISCO, Riccardo Thefile contains technical information submitted after I-28069 Trecate (IT) the application was filed and not included in this specification (74) Representative: Palladino, Saverio Massimo et al Notarbartolo & Gervasi S.p.A. -

Hormone Replacement Therapy and Osteoporosis
This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. AHRQ is the lead Federal agency charged with supporting research designed to improve the quality of health care, reduce its cost, address patient safety and medical errors, and broaden access to essential services. AHRQ sponsors and conducts research that provides evidence-based information on health care outcomes; quality; and cost, use, and access. The information helps health care decisionmakers— patients and clinicians, health system leaders, and policymakers—make more informed decisions and improve the quality of health care services. Systematic Evidence Review Number 12 Hormone Replacement Therapy and Osteoporosis Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 2101 East Jefferson Street Rockville, MD 20852 http://www.ahrq.gov Contract No. 290-97-0018 Task Order No. 2 Technical Support of the U.S. Preventive Services Task Force Prepared by: Oregon Health Sciences University Evidence-based Practice Center, Portland, Oregon Heidi D. Nelson, MD, MPH August 2002 Preface The Agency for Healthcare Research and Quality (AHRQ) sponsors the development of Systematic Evidence Reviews (SERs) through its Evidence-based Practice Program. With guidance from the third U.S. Preventive Services Task Force∗ (USPSTF) and input from Federal partners and primary care specialty societies, two Evidence-based Practice Centers—one at the Oregon Health Sciences University and the other at Research Triangle Institute-University of North Carolina—systematically review the evidence of the effectiveness of a wide range of clinical preventive services, including screening, counseling, immunizations, and chemoprevention, in the primary care setting. -

Pharmacology/Therapeutics II Block III Lectures 2013-14
Pharmacology/Therapeutics II Block III Lectures 2013‐14 66. Hypothalamic/pituitary Hormones ‐ Rana 67. Estrogens and Progesterone I ‐ Rana 68. Estrogens and Progesterone II ‐ Rana 69. Androgens ‐ Rana 70. Thyroid/Anti‐Thyroid Drugs – Patel 71. Calcium Metabolism – Patel 72. Adrenocorticosterioids and Antagonists – Clipstone 73. Diabetes Drugs I – Clipstone 74. Diabetes Drugs II ‐ Clipstone Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones Date: Thursday, March 20, 2014-8:30 AM Reading Assignment: Katzung, Chapter 37 Key Concepts and Learning Objectives To review the physiology of neuroendocrine regulation To discuss the use neuroendocrine agents for the treatment of representative neuroendocrine disorders: growth hormone deficiency/excess, infertility, hyperprolactinemia Drugs discussed Growth Hormone Deficiency: . Recombinant hGH . Synthetic GHRH, Recombinant IGF-1 Growth Hormone Excess: . Somatostatin analogue . GH receptor antagonist . Dopamine receptor agonist Infertility and other endocrine related disorders: . Human menopausal and recombinant gonadotropins . GnRH agonists as activators . GnRH agonists as inhibitors . GnRH receptor antagonists Hyperprolactinemia: . Dopamine receptor agonists 1 Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. 1. Overview of Neuroendocrine Systems The neuroendocrine -

Ethynylestradioland Moxestrolin Normalandtumor-Bearingrats
RadiotracersBindingto EstrogenReceptors:I: TissueDistributionof 17a- EthynylestradiolandMoxestrolin NormalandTumor-BearingRats A. Feenstra,G.M.J. Nolten,W.Vaalburg,S. Reiffers,andM.G.Woldring University Hospital, Groningen, TheNetherlands Ethynylestradioland moxestroican be labeled with carbon-i 1 by introducIng thisposItronemitter in the 17a-ethynyl group.To investigatetheir potentialas ra diotracersbIndIngto estrogenreceptors,we studiedthe tIssuedistributionof tn tiated ethynylestradloland moxestrol,with specific activItIesof 57 CI/mmol and 77-90 Ci/mmol, respectively, In the adult female rat. At 30 mm after injection, both compoundsshowedspecificuptake in the uterus( % dose/g): 2.52 for ethynyles tradiol and of 2.43 for moxestrol.A decrease of the specific activIty to 6-9 CI/ mmolresultedinuterineuptakesof 1.60 and2.iO respectively,forethynylestradlol and moxestrol,at 30 mm. In the female rat bearingDMBA-Inducedmammarytu mors,specIficuptakewas alsomeasuredin the tumors,althoughthe valueswere only25-30% ofthe uterineuptake.Moxestrolshoweda greateruptakeselectivity in the tumorscomparedwith ethynylestradlol.From this studywe concludethat ethynylestradiolandmoxestrolhavegoodpotentialastracersbIndingto mammary tumorsthat contaInestrogenreceptors. J Nucl Med 23: 599—605,1982 During the last decade the relationship between the mination has evoked much interest in developing ra estrogen-receptor concentration and the response to diopharmaceuticals capable of binding to the estrogen endocrine therapy in human breast cancer has become receptor -

A Pharmaceutical Product for Hormone Replacement Therapy Comprising Tibolone Or a Derivative Thereof and Estradiol Or a Derivative Thereof
Europäisches Patentamt *EP001522306A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 306 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: A61K 31/567, A61K 31/565, 13.04.2005 Bulletin 2005/15 A61P 15/12 (21) Application number: 03103726.0 (22) Date of filing: 08.10.2003 (84) Designated Contracting States: • Perez, Francisco AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 08970 Sant Joan Despi (Barcelona) (ES) HU IE IT LI LU MC NL PT RO SE SI SK TR • Banado M., Carlos Designated Extension States: 28033 Madrid (ES) AL LT LV MK (74) Representative: Markvardsen, Peter et al (71) Applicant: Liconsa, Liberacion Controlada de Markvardsen Patents, Sustancias Activas, S.A. Patent Department, 08028 Barcelona (ES) P.O. Box 114, Favrholmvaenget 40 (72) Inventors: 3400 Hilleroed (DK) • Palacios, Santiago 28001 Madrid (ES) (54) A pharmaceutical product for hormone replacement therapy comprising tibolone or a derivative thereof and estradiol or a derivative thereof (57) A pharmaceutical product comprising an effec- arate or sequential use in a method for hormone re- tive amount of tibolone or derivative thereof, an effective placement therapy or prevention of hypoestrogenism amount of estradiol or derivative thereof and a pharma- associated clinical symptoms in a human person, in par- ceutically acceptable carrier, wherein the product is pro- ticular wherein the human is a postmenopausal woman. vided as a combined preparation for simultaneous, sep- EP 1 522 306 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 1 522 306 A1 2 Description [0008] The review article of Journal of Steroid Bio- chemistry and Molecular Biology (2001), 76(1-5), FIELD OF THE INVENTION: 231-238 provides a review of some of these compara- tive studies.