Cochlear Morphology in the Developing Inner Ear of the Porcine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cochlear Implants in Unilateral Hearing Loss for Tinnitus Suppression
Central Annals of Otolaryngology and Rhinology Review Article *Corresponding author Mohamed Salah Elgandy, Department of Otolaryngology-Head and Neck Surgery, University of Cochlear Implants in Unilateral Iowa hospital and clinics, 200 Hawkins drive, Iowa, Iowa City 52242.PFP 21167, USA, Tel; +1(319)519-3862; Email; Hearing Loss for Tinnitus Submitted: 19 January 2019 Accepted: 06 February 2019 Suppression Published: 08 February 2019 ISSN: 2379-948X 1,2 2,3 Mohamed Salah Elgandy *and Richard S. Tyler Copyright 1Department of Otolaryngology-Head and Neck Surgery, Zagazig University, Egypt © 2019 Elgandy et al. 2Department of Otolaryngology-Head and Neck Surgery, University of Iowa, USA 3Department of Communication Sciences and Disorders, University of Iowa, USA OPEN ACCESS Keywords Abstract • Unilateral hearing loss Tinnitus is a pervasive symptom that can affect many people with hearing loss. • Tinnitus It is found that its incidence is increasing due to accompanying occupational and • Electrical stimulation environmental noise. Even, there is no standard treatment is present up till now, but • Cochlear implants cochlear implants (CIs) positive effects are well proven and documented. This article provides an overview of many publicly available reports about cochlear implants and tinnitus, with review of several articles demonstrating the benefit of cochlear implants for unilateral hearing loss and tinnitus. We believe that this approach will help many, and should be considered as standard practice and reimbursed. INTRODUCTION An increase in rate. Unilateral hearing loss affecting approximately18.1 million • Decrease in rate persons in the United States [1]. Patients with unilateral deafness • Periodic activity frequently also experience tinnitus, which can have a profound • Synchronous activity cross neurons has been associated with an increased incidence of depression, impact on an individual’s quality of life. -

Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss
Technology Assessment Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss Technology Assessment Program Prepared for: Original: April 11, 2011 Agency for Healthcare Correction: June 17, 2011 Research and Quality 540 Gaither Road Rockville, Maryland 20850 i Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss Technology Assessment Report Project ID: AUDT0510 Original date: April 11, 2011 Correction date: June 17, 2011 * See Errata document for a summary of corrections. Tufts Evidence-based Practice Center Gowri Raman, M.D., M.S. Jounghee Lee, PhD Mei Chung, PhD, MPH James M. Gaylor, BA Srila Sen, M.A. (Editor) Madhumathi Rao, M.D., PhD Joseph Lau, M.D. Technical Consultants Dennis S. Poe, M.D. Associate Professor, Dept of Otology & Laryngology Harvard Medical School, Boston Marilyn W. Neault, PhD, CCC-A Director, Habilitative Audiology Program Children's Hospital, Boston ii This report is based on research conducted by the Tufts Evidence-based Practice Center under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290 2007 10055 1). The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decision-makers; patients and clinicians, health system leaders, and policymakers, make well- informed decisions and thereby improve the quality of health care services. -

Cochlear Implant Guide
Cochlear Implant Guide Ear & Hearing Center at Texas Children’s Hospital® Dear Families, Welcome to the Cochlear Implant Program at Texas Children’s Hospital! Our goal is to provide comprehensive, specialized care for children with severe hearing loss, and help them achieve the best possible communication outcomes. Since our program started in 2001, we have performed more than 700 implants. Your child may be a candidate for a cochlear implant in one or both ears. Cochlear implants, or CIs, are innovative devices which can directly stimulate the auditory nerve and provide access to sound in children with severe or profound hearing loss. The decision about whether to recommend a CI is highly individualized, and requires the input of specialists in multiple areas, including Otolaryngology, Audiology, Speech Pathology, and Neuropsychology, among others. These specialists make up the members of the Cochlear Implant Team. They work in close collaboration to determine who is a candidate for a CI, perform the implantation surgery, and help patients through the rehabilitation afterwards. This guide will help you understand the entire process, who may be considered a CI candidate, the basics of how the device works, how the surgery is done, and the necessary rehabilitation to achieve a successful outcome. Thank you for the opportunity to help your family through this journey. All of our team members are dedicated to helping children with hearing loss achieve their full potential. If you have any questions or concerns, please do not hesitate to contact us. Sincerely, The Cochlear Implant Team at Texas Children’s Hospital Table of Contents 1. -

Sensori-Neural Deafness and the Cochlear Implant
Rochester Institute of Technology RIT Scholar Works Theses 9-30-1997 Sensori-neural deafness and the cochlear implant Margaret Pence Follow this and additional works at: https://scholarworks.rit.edu/theses Recommended Citation Pence, Margaret, "Sensori-neural deafness and the cochlear implant" (1997). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. Approvals Chief Advisor: Glen Hintz Date Associate Advisor: Robert Wabnitz Date : i - /] - q'} Associate Advisor: Catherine Clark Date /~J I '/ f? Associate Advisor: Dr. Paul Dutcher Date /LO/77 Chairperson: Tom Lightfoot Date / 7 2-/;I/?~ 1 I I, Margaret Pence, hereby grant permission to the Wallace Memorial Library of RIT to reproduce my thesis in whole or in part. Any reproduction will not be for commercial use or profit. Signature Date Rochester Institute of Technology A Thesis submitted to the Faculty of the College of Imaging Arts and Sciences in candidacy for the degree of Master of Fine Arts. Sensori-neural Deafness and the Cochlear Implant by Margaret Pence 9/30/97 Introduction The inner ear is part of an intricate network of fluid filled cavities located in the temporal bone of the skull. Within this bony labyrinth is the cochlea which houses the organ of hearing called the organ of Corti. Any number of factors, including disease, trauma and congenital malformations may interrupt the processing of sound through the inner ear. -

Prombera: a Preoperative Eabr: an Update
Current Directions in Biomedical Engineering 2018; 4(1): 563 – 565 Daniel Polterauer*, Giacomo Mandruzzato, Maike Neuling, Marek Polak, Joachim Müller, John-Martin Hempel PromBERA: A preoperative eABR: An update Objective promontory stimulation eABR test for measuring the integrity of the auditory nerve in prospective CI candidates. Abstract: Prior to cochlear implantation, audiological https://doi.org/10.1515/cdbme-2018-0135 tests are performed to determine candidacy in subjects with a hearing loss. This is usually done by measuring the acoustic auditory brainstem response (ABR). 1 Introduction Unfortunately, for some subjects, a reproducible ABR recording cannot be obtained, even at high acoustic levels. Promontory Stimulation is a well-established tool to Having a healthy stimulating auditory nerve is stimulate the cochlea preoperatively with a temporary required for cochlear implantation in order to benefit from transtympanic needle placed in the middle ear [1]. It has the electrical pulses that are generated by the implant and been shown that electrically evoked Auditory Brainstem to improve speech comprehension. In some subjects, this Response (eABR) measurements recorded with prerequisite cannot be measured using routine Promontory Stimulation is an useful objective audiological tests. measurement in cochlear implant (CI) candidates for In this study, the feasibility of recording testing and evaluating the presence and excitability of the electrically evoked auditory brainstem responses (eABR) auditory nerve and auditory pathway before cochlear using a stimulating transtympanic electrode, placed on the implantation [2]. This test is especially important for round window niche, together with MED-EL clinical subjects where it is difficult or not possible to determine system is investigated. -

Cochlear Implants
Cochlear Implants A cochlear implant is an electronic device that restores partial hearing to individuals with severe to profound hearing loss who do not benefit from a conventional hearing aid. It is surgically implanted in the inner ear and activated by a device worn outside the ear. Unlike a hearing aid, it does not make sound louder or clearer. Instead, the device bypasses damaged parts of the auditory system and directly stimulates the nerve of hearing, allowing individuals who are profoundly hearing-impaired to receive sound. What is normal hearing? Your ear consists of three parts that play a vital role in hearing—the external ear, middle ear, and inner ear. Conductive hearing: Sound travels along the ear canal of the external ear, causing the ear drum to vibrate. Three small bones of the middle ear conduct this vibration from the eardrum to the cochlea (auditory chamber) of the inner ear. Sensorineural hearing: When the three small bones move, they start waves of fluid in the cochlea, and these waves stimulate more than 16,000 delicate hearing cells (hair cells). As these hair cells move, they generate an electrical current in the auditory nerve. The electrical signal travels through inter-connections in the brain to specific areas of the brain that recognize it as sound. How is hearing impaired? If you have disease or obstruction in your external or middle ear, your conductive hearing may be impaired. Medical or surgical treatment can probably correct this. An inner ear problem, however, can result in a sensorineural impairment, or nerve deafness. In most cases, the hair cells are damaged and do not function. -

Cochlear Implants
AUDIOLOGY INFORMATION SERIES Cochlear Implants ……………………………………………………………………………………………………………………………………………………………………………………………………… What is a cochlear implant? profound hearing loss in that ear. Candidacy for an A cochlear implant is a device that helps people with implant is based on the results of audiometric tests significant hearing loss. It will not provide “normal” that examine the severity of the hearing loss and the hearing. However, it can provide recipients with patient’s ability to recognize speech with and important access to auditory information. This may without hearing aids. lead to greatly improved communication and A team of professionals will work together to see if a awareness of environmental sounds. The first cochlear implant will work for you. This team may cochlear implant was introduced in 1972. The U.S. include the following people: Food and Drug Administration (FDA) has found them an audiologist to be a safe and effective treatment for hearing loss. an ear, nose, and throat doctor or surgeon a neuropsychologist a counselor or social worker How does a cochlear implant work? a speech-language pathologist (SLP) A cochlear implant has parts placed both on the teachers, as needed outside and inside of your head. These parts work together to help you tune into sounds. Everyone on the team has a different role to help External, or outside, parts: You will have a you. The implant surgeon will perform a medical device that looks like a hearing aid connected to a evaluation. This includes radiographic testing to magnet above the same ear on the side of your evaluate the status of the inner ear. CT scans and head. -

Cochlear Implants Answers to Your Questions
Cochlear Implants Answers to your questions 1 Welcome This guide is designed to give you the information you need when considering a cochlear implant for yourself or someone important in your life. It will help you understand how hearing works, hearing with a cochlear implant and answer some common questions about getting a cochlear implant. YOU’RE NOT ALONE Hundreds of millions of people around the world experience some form of hearing loss. While many can be helped by a hearing aid, more than 75 million people still find the most powerful hearing aids inadequate. DID YOU KNOW? More than 170,000 people around the world have received a Nucleus® cochlear implant. 2 3 How do our ears work? The ear is an amazingly complex but efficient hearing system. The ear is made up of three sections: The whole process only takes OUTER MIDDLE INNER a fraction of a second. Ossicles How do we hear? 3 1 Sound waves are guided down your ear canal by the pinna. Pinna 1 4 2 The sound waves hit your ear drum and it vibrates. 2 Cochlea 3 The small ossicles vibrate with the ear drum, transferring the sound across the middle ear to the cochlea. Ear Drum 4 The fluid inside the cochlea picks up the vibrations and carries them to the thousands of tiny hair cells. These hair cells change the movement into OUTER MIDDLE INNER electrical impulses which are sent The outer ear consists of the pinna The middle ear consists of the ear The inner ear consists of a complicated along the auditory and the ear canal. -

Cochlear and Auditory Brainstem Implants
Medical Coverage Policy Effective Date ............................................11/15/2020 Next Review Date ......................................11/15/2021 Coverage Policy Number .................................. 0190 Cochlear and Auditory Brainstem Implants Table of Contents Related Coverage Resources Overview .............................................................. 1 Hearing Aids Coverage Policy ................................................... 1 Otoplasty and External Ear Reconstruction General Background ............................................ 4 Speech Therapy Medicare Coverage Determinations .................. 18 Coding/Billing Information .................................. 18 References ........................................................ 20 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies -
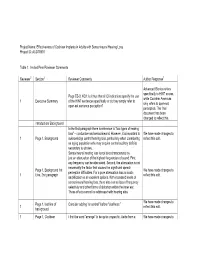
Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss Project ID: AUDT0501
Project Name: Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss Project ID: AUDT0501 Table 1: Invited Peer Reviewer Comments Reviewer1 Section2 Reviewer Comments Author Response3 Advanced Bionics refers specifically to HINT scores, Page ES-3, KQ1: Is it true that all CI indications specify the use while Cochlear Americas 1 Executive Summary of the HINT sentences specifically or do they simply refer to only refers to open-set open-set sentence perception? perception. The final document has been changed to reflect this. Introduction/ Background In the first paragraph there is reference to “two types of hearing loss” – conductive and sensorineural. However, it is important to We have made changes to 1 Page 1, Background acknowledge central hearing loss, particularly when considering reflect this edit. an aging population who may acquire central auditory deficits secondary to strokes. Sensorineural hearing loss is not best characterized by just an attenuation of the highest frequencies of sound. First, any frequency can be attenuated. Second, the attenuation is not necessarily the factor that causes the significant speech Page 1, Background 1st We have made changes to perception difficulties. For a pure attenuation loss acoustic 1 Line, 2nd paragraph reflect this edit. amplification is an excellent options. With increased levels of sensorineural hearing loss, there also comes loss of frequency selectivity and other forms of distortion within the inner ear. These effects cannot be addressed with hearing aids. We have made changes to Page 1, last line of Consider adding “or central” before “deafness.” 1 reflect this edit. background 1 Page 1, Cochlear I find the word “arrange” to be quite unspecific. -

Frequently Asked Questions
Northwestern Medicine Cochlear Implant Program for Adults FREQUENTLY ASKED QUESTIONS Cochlear Implants The following information refers to cochlear implants for adults. Although many parts of the discussion are also applicable to children, the evaluation process and expected outcomes sections are specifically geared toward adults. What is a cochlear implant? How does a cochlear implant work? How is a cochlear implant different from a hearing aid? Who is a candidate for a cochlear implant? What is the evaluation process for getting a cochlear implant? Who manufactures cochlear implants? What do I do if I think I’m a cochlear implant candidate? Who does the surgery for a cochlear implant? What risks are associated with cochlear implant surgery? How soon after surgery does the patient hear with a cochlear implant? What happens during device “activation?” How much benefit does a patient receive from a cochlear implant? What if a better implant is developed after I get mine? Do people with cochlear implants continue to use a hearing aid on the non-implant side? What about two cochlear implants—one for each ear? Does a cochlear implant user ever stop learning to hear better? What is a cochlear implant? A cochlear implant provides a perception of sound to those who would not otherwise have access to sound. The implant changes sounds into electrical pulses which stimulate Northwestern Cochlear Implant Program February, 2016 the auditory (hearing) nerve directly. The auditory nerve sends these coded electrical pulses to the brain, which is where we really “hear.” The cochlear implant device has two pieces. One piece (internal receiver/stimulator) is implanted into the inner ear during a surgical procedure. -

Introduction to CI (Cochlear Implant)
Phonak + AB resource Introduction to CI (Cochlear Implant) 2019 - This resource was created by the first Phonak Teen Advisory Board. What is a Cochlear Implant? A cochlear implant (CI) is a type of surgically implanted hearing technology. CIs can improve clarity of sounds, music, and your ability to hear your friends and others talking. CIs can help you understand and connect to your world better and improve your ability to hear in noisy situations. What are the components of a Cochlear Implant? A cochlear implant system consists of an internal device and an external sound processor. microphone External device Internal device sound processor headpiece electrode array magnet battery Follow the graphic below beginning with number 1 to learn how a cochlear implant works. How does a cochlear implant help connect you to the world around you? You can connect your cochlear implant to your phone, tablet, computer, gaming headset, or Bluetooth® devices. You can also stream music and movies to your cochlear implant. How does a cochlear implant differ from a hearing aid? There are many similarities and differences between a hearing aid (HA) and a cochlear implant (CI). From a benefits perspective, both will help you to hear the world around you whether that be hearing conversations, hearing music or helping to keep you safe. From a hardware perspective, both have a microphone that picks up the sound and then the hearing aid or cochlear implant prepares the sound before delivering it to the hearing nerve so that the information can be used by the person with hearing loss.