Heat Shock Induction of a 65 Kda ATP-Binding Proteinase in Rat C6 Glioma Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silk Road Fashion, China. the City and a Gate, the Pass and a Road – Four Components That Make Luoyang the Capital of the Silk Roads Between 1St and 7Th Century AD
https://publications.dainst.org iDAI.publications ELEKTRONISCHE PUBLIKATIONEN DES DEUTSCHEN ARCHÄOLOGISCHEN INSTITUTS Dies ist ein digitaler Sonderdruck des Beitrags / This is a digital offprint of the article Patrick Wertmann Silk Road Fashion, China. The City and a Gate, the Pass and a Road – Four components that make Luoyang the capital of the Silk Roads between 1st and 7th century AD. The year 2018 aus / from e-Forschungsberichte Ausgabe / Issue Seite / Page 19–37 https://publications.dainst.org/journals/efb/2178/6591 • urn:nbn:de:0048-dai-edai-f.2019-0-2178 Verantwortliche Redaktion / Publishing editor Redaktion e-Jahresberichte und e-Forschungsberichte | Deutsches Archäologisches Institut Weitere Informationen unter / For further information see https://publications.dainst.org/journals/efb ISSN der Online-Ausgabe / ISSN of the online edition ISSN der gedruckten Ausgabe / ISSN of the printed edition Redaktion und Satz / Annika Busching ([email protected]) Gestalterisches Konzept: Hawemann & Mosch Länderkarten: © 2017 www.mapbox.com ©2019 Deutsches Archäologisches Institut Deutsches Archäologisches Institut, Zentrale, Podbielskiallee 69–71, 14195 Berlin, Tel: +49 30 187711-0 Email: [email protected] / Web: dainst.org Nutzungsbedingungen: Die e-Forschungsberichte 2019-0 des Deutschen Archäologischen Instituts stehen unter der Creative-Commons-Lizenz Namensnennung – Nicht kommerziell – Keine Bearbeitungen 4.0 International. Um eine Kopie dieser Lizenz zu sehen, besuchen Sie bitte http://creativecommons.org/licenses/by-nc-nd/4.0/ -

World Bank Document
CONFORMED COPY LOAN NUMBER 7909-CN Public Disclosure Authorized Project Agreement Public Disclosure Authorized (Henan Ecological Livestock Project) between INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT Public Disclosure Authorized and HENAN PROVINCE Dated July 26, 2010 Public Disclosure Authorized PROJECT AGREEMENT AGREEMENT dated July 26, 2010, entered into between INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT (the “Bank”) and HENAN PROVINCE (“Henan” or the “Project Implementing Entity”) (“Project Agreement”) in connection with the Loan Agreement of same date between PEOPLE’S REPUBLIC OF CHINA (“Borrower”) and the Bank (“Loan Agreement”) for the Henan Ecological Livestock Project (the “Project”). The Bank and Henan hereby agree as follows: ARTICLE I – GENERAL CONDITIONS; DEFINITIONS 1.01. The General Conditions as defined in the Appendix to the Loan Agreement constitute an integral part of this Agreement. 1.02. Unless the context requires otherwise, the capitalized terms used in the Project Agreement have the meanings ascribed to them in the Loan Agreement or the General Conditions. ARTICLE II – PROJECT 2.01. Henan declares its commitment to the objective of the Project. To this end, Henan shall: (a) carry out the Project in accordance with the provisions of Article V of the General Conditions; and (b) provide promptly as needed, the funds, facilities, services and other resources required for the Project. 2.02. Without limitation upon the provisions of Section 2.01 of this Agreement, and except as the Bank and Henan shall otherwise agree, Henan shall carry out the Project in accordance with the provisions of the Schedule to this Agreement. ARTICLE III – REPRESENTATIVE; ADDRESSES 3.01. -

Acquisition of Land Use Rights in Shangqiu City, Henan Province, the Prc
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. ZENSUN ENTERPRISES LIMITED 正 商 實 業 有 限 公 司 (Incorporated in Hong Kong with limited liability) (Stock Code: 185) VOLUNTARY ANNOUNCEMENT ACQUISITION OF LAND USE RIGHTS IN SHANGQIU CITY, HENAN PROVINCE, THE PRC This is a voluntary announcement made by Zensun Enterprises Limited (“Company”). The board (“Board”) of directors (“Directors”) of the Company is pleased to announce that on 26 May 2021, Henan Zensun Zhengxin Real Estate Company Limited* (河南正商鄭新房 地產有限公司) (“Henan Zhengxin”), an indirect wholly owned subsidiary of the Company, made a successful bid for the transfer of state-owned land use rights of a land parcel with code no. 2020-68 (the “Land Parcel”) located in Yucheng County, Shangqiu City, Henan Province, the People’s Republic of China (the “PRC”) through listing for sale process (the “Acquisition”) in the public auction (“Auction”) held by Yucheng County Natural Resources Bureau* (虞城縣自然資源局) at a consideration of RMB111,820,000 for the Land Parcel. The consideration of the Acquisition was determined based on the Auction documents issued by Yucheng County Natural Resources Bureau. The Group will finance the Acquisition and the development of the Land Parcel with internal resources. The Land Parcel is located at north of Songshan Road* (嵩山路北側), Yucheng County, Shangqiu City, Henan Province, the PRC with a total site area of approximately 51,410.06 sq.m. -

Shang Beyond Anyang *2. an JINHUAI (Henan Institute of Cultural
Shang Beyond Anyang *2. AN JINHUAI (Henan Institute of Cultural Objects, Zhengzhou) THE SHANG CITY AT ZHENGZHOU AND RELATED PROBLEMS ABSTRACT: The Zhengzhou Shang dynasty site is the location of an early Shang city, vast in area and abundant in archaeological remains, which was discovered by Chinese archaeologists in the middle and lower Yellow River basin during the early fifties. Within the site there is a Shang dynasty rammed-earth wall extending north-south in a rectangular shape and having a circumference of 6960 meters. These are the earliest Shang wall remains discovered to date. Based on the stratigraphy and vessel types discovered in the course of excavating the four sides of the wall, it is certain that this wall is slightly later than the late Erlitou period, and that construction on it began before the lower strata of the Shang Erligang period, the "Yin Ruins" at Anyang. The discovery of the Zhengzhou Shang site was definitely not accidental. It represents an important stage in the development of ancient Chinese rammed-earth wall architecture. The method of construction places it in a contin uous line of development from the rammed-earth wall of the Henan area middle and late Longshan culture and the late Erlitou rammed-earth platform foundation to the rammed-earth foundations of the palaces of the Yin Ruins at Anyang. The grand scale of the Zhengzhou Shang wall, and the fact that inside and outside the wall were found palace foundations and work shops for the production of bronze, bone, and ceramic articles as well as numerous widespread storage pits, wells, ditches, house foundations, and tombs, and that many bronze, jade, primitive porcelain, pottery, stone, bone, and clamshell artifacts have been excavated here, including also some carved ivory pieces, pottery sculpture, and inscribed bones and pottery, lead us to conclude that the Zhengzhou Shang site was one of the early Shang capitals. -

Lauren Ledin Anyang, China Field School 2012 Early This Summer, I
Lauren Ledin Anyang, China Field School 2012 Early this summer, I participated in a six-week field school in China. It was a combination of training in field survey and excavation, conducting experimental archaeology, and travel to relevant archaeological sites. The first week we stayed in Beijing. During a visit to the National Museum of China we saw artifacts from the time period which we were focusing on-- the Late Shang period. This proved to be very helpful in giving us a background of information and examples of artifacts dated earlier and later than the Late Shang period. Zhoukoudian, the site of the Peking man, was our next stop in Beijing Province. Later in the week, we attended an archaeological conference and were able to join in meeting and honoring Zheng Zhenxiang, the team leader of Fu Hao's tomb excavation in Anyang. We also managed to fit in a tour of The Forbidden City and a visit to the Great Wall. Other places we traveled to included Xi’an and the surrounding areas where we saw the Terracotta Army in Lishan, the Xi’an city wall, and also the beautiful mixing of cultures at the Xi’an Mosque. Within Henan Province we ventured to a Sanmenxia museum highlighting the Western Zhou State of Guo, to the Taihang Mountains in search of sandstone sources used as abrasives in jade carving, and to the Neolithic site of Xiao Dong Nan Xue Yi Zhi. Anyang City itself is home to the National Museum of Chinese Writing, the Yin Ruins, and the Royal Cemetery, all of which we visited. -

Anyang Wastewater Treatment & Water Supply Project Under Henan
RESETTLEMENT PLAN Anyang Wastewater Treatment & Water Supply Project Under Henan Wastewater Management and Water Supply Sector Project IN THE PEOPLE’S REPUBLIC OF CHINA Anyang Municipal Water Supply Company April 2005 THIS IS NOT AN ADB BOARD APPROVED DOCUMENT 1 Endorsement Letter of the Resettlement Plan Anyang Water Affairs General Company has prepared the resettlement plan for Asian Development Bank (ADB) financed wastewater treatment project in our city. This resettlement plan fully complies with requirements of the relevant laws, regulations and policies of People’s Republic of China and Henan Province as well as complies with ADB’s policy on involuntary resettlement. Anyang Municipal Government hereby confirms the content of this resettlement plan and will guarantee the land acquisition, compensation and relocation budget being provided according to the provisions of this resettlement plan. This resettlement plan is based on the feasibility study report and the initial surveys. If the final implemented engineering works are different from what have been described in the feasibility study report and that will cause the substantial impact on the resettlement plan, this resettlement plan should be future modified and approved by ADB before its implementation. Anyang Municipal People’s Government March 28, 2005 2 ABBREVIATIONS RP Resettlement Plan APs Affected Persons ADB Asian Development Bank WWTPLT Anyang Zongcun Wastewater Treatment subproject Leading Team PMO Project Management Office AYWAG Anyang Water Affairs Group PPO Zongcun -
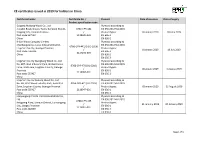
CE Certificates Issued in 2019 for Holders in China
CE certificates issued in 2019 for holders in China Certificate holder Certificate No. / Product Date of issuance Date of expiry Product specification code Guigang Huidong Wood Co., Ltd. Plywood according to Jiangbei Road, Qiaoxu Town, Gangnan District, 0766-CPR-481 EN 636:2012+A1:2015 Guigang City, Guanxi Province / Product types: 8 January 2019 18 June 2019 Post code 537132 2118049-002 EN 636-1 China EN 636-2 K-Sun Wood Company Limited Plywood according to Chachangpianqu, Luwu Industrial District, EN 636:2012+A1:2015 0766-CPR-441/1 (01-2019) Lingshan County, Guangxi Province Product types: / 8 January 2019 19 July 2019 Post code: 535416 EN 636-1 2117235-003 China EN 636-2 EN 636-3 Lingshan County Qiangliang Wood Co., Ltd. Plywood according to No. B07, Wuli Industrial Park, Qinlian Forest EN 636:2012+A1:2015 0766-CPR-470 (01-2019) Farm, WuliTown, Lingshan County, Guangxi Product types: / 8 January 2019 5 August 2019 Province EN 636-1 2118066-001 Post code 535427 EN 636-2 China Lingshan County Guitong Wood Co., Ltd. Plywood according to No. A9, Wuli Wood Industry Park, Industrial 0766-CPR-471 (01-2019) EN 636:2012+A1:2015 Zone, Lingshan County, Guangxi Province / Product types: 8 January 2019 12 August 2019 Post code 535427 2118047-001 EN 636-1 China EN 636-2 Lianyungang Chanta International Wood Co., Plywood according to Ltd. EN 636:2012+A1:2015 0766-CPR-485 Kangpeng Plaza, Lianyun District, Lianyungang Product types: / 21 January 2019 20 January 2020 City, Jiangsu Province EN 636-1 2119007-001 Post code 222000 EN 636-2 China EN 636-3 Page 1 of 8 CE certificates issued in 2019 for holders in China Certificate holder Certificate No. -

China's Judicial System and Judicial Reform Nicholas C
University of Michigan Law School University of Michigan Law School Scholarship Repository Other Publications Faculty Scholarship 2010 China's Judicial System and Judicial Reform Nicholas C. Howson University of Michigan Law School, [email protected] Available at: https://repository.law.umich.edu/other/3 Follow this and additional works at: https://repository.law.umich.edu/other Part of the Comparative and Foreign Law Commons, Courts Commons, and the Rule of Law Commons Recommended Citation Howson, Nicholas C. (2005- ). "China's Judicial System and Judicial Reform." Law Quad. Notes 54, no. 1 (2011): 62-4. (This text is an extract from the statement delivered by Professor Howson at the inaugural "China-U.S. Rule of Law Dialogue" held at Beijing's Tsinghua University, July 29-30, 2010.) This Speech is brought to you for free and open access by the Faculty Scholarship at University of Michigan Law School Scholarship Repository. It has been accepted for inclusion in Other Publications by an authorized administrator of University of Michigan Law School Scholarship Repository. For more information, please contact [email protected]. FacULTY VIEWS China’s Judicial System and Judicial Reform The following is an extract from the statement delivered by Michigan Law School Professor Nicholas Howson at the inaugural “China-U.S. Rule of Law Dialogue” held at Beijing’s Tsinghua University July 29-30, 2010, and convened by Tsinghua Law Dean Wang Zhenmin and Harvard Law School Professor and East Asian Legal Studies Director William Alford, and with the COURTESY OF COURTESY support of the China-United States Exchange Foundation chaired by C.H. -

Spatio-Temporal Evolution and Prediction of Tourism Comprehensive Climate Comfort in Henan Province, China
atmosphere Article Spatio-Temporal Evolution and Prediction of Tourism Comprehensive Climate Comfort in Henan Province, China Junyuan Zhao 1 and Shengjie Wang 2,* 1 School of Business, Xinyang College, West Section of Xinqi Avenue, Shihe District, Xinyang 464000, China; [email protected] 2 College of Geography and Environment Science, Northwest Normal University, No. 967, Anning East Road, Lanzhou 730070, China * Correspondence: [email protected] Abstract: The tourism comprehensive climate comfort index (TCCI) was used to evaluate the tourism climate comfort in Henan Province in the last 61 years, and its future development trend is predicted. The results showed that the temporal variation of the TCCI had a “double peak” type (monthly variation), and an overall comfort improvement trend (interannual variation). The change of tourism climate comfort days was similar to the change of the index, especially in the months with a low comfort level. In space, the distribution of the TCCI gradually increased from northeast to southwest, and the area with a high comfort level also increased over time. Meanwhile, it also showed the spatial distribution of months with a low comfort level, which provides reliable information for tourists to use when choosing tourist destinations across all periods of the year. The TCCI was classified by hierarchical classification, and principal components were extracted to explore the main climate factors controlling different types of TCCIs and the relationship between them, and Citation: Zhao, J.; Wang, S. large-scale atmospheric–oceanic variability. According to the temporal change trend and correlation, Spatio-Temporal Evolution and the long-term change trend of tourism climate comfort was predicted, which will provide a scientific Prediction of Tourism Comprehensive basis for tourism planners to choose tourist destinations. -

Degruyter Gps Gps-2020
Nucleotide-based green synthesis of lanthanide coordination polymers I Research Article Yaoyao Zhang, Baoxia Liu*, Qi Shen, Xiuhua Wei, Yanli Zhou, Yintang Zhang, Peng Qu, and Maotian Xu Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission C (1) AMP/Tb-CIP C O Gd (3) AMP/Gd-BTC O P Tb P N N Intensity (a.u.) Intensity Intensity (a.u.) Intensity Tb Tb F Gd P P F Tb Gd Gd 012345678012345678 Energy (KeV) Energy (KeV) C (2) AMP/Eu-BTC C (4) AMP/Tb Eu Gd -CIP P 0.1 0.9 99.0 O O N Tb, Eu, Gd P Eu N Intensity (a.u.) Tb, Eu, Gd F Eu F Intensity (a.u.) Intensity P P Eu Tb, Eu, Gd 012345678 012345678 Energy (KeV) Energy (KeV) Figure S1: Energy-dispersive X-ray (EDX) spectra of (1) AMP/Tb-CIP, (2) AMP/Eu-CIP, (3) AMP/Gd-CIP, and (4) AMP/Tb0.1Eu0.9Gd99.0-CIP. Q1 * Corresponding author: Baoxia Liu, Henan Key Laboratory of Biomolecular Recognition and Sensing, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, P. R. China; Henan Joint International Research Laboratory of Chemo/ Biosensing and Early Diagnosis of Major Diseases, Shangqiu Normal University, Shangqiu, 476000, Henan Province, PR China, e-mail: [email protected], tel: +86 370 2595623, fax: +86 370 2595623 Yaoyao Zhang: Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou 450001, China; Henan Key Laboratory of Biomolecular Recognition and Sensing, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, P. -

Quotation Sheet
Zhengzhou Changli Machinery Manufacturing Co., Ltd. 50 meters to the East of Century Plaza, Shangjie District Zhengzhou City, Henan, China. Contact person: Ivy Tel: +86-371-66985358 Fax: +86-371-66987958 Skype :ivy.han6 Email: [email protected] QUOTATION SHEET JS1000 Twin Shaft Concrete Mixer Zhengzhou Changli Machinery Manufacturing Co., Ltd. 50 meters to the East of Century Plaza, Shangjie District Zhengzhou City, Henan, China. Contact person: Ivy Tel: +86-371-66985358 Fax: +86-371-66987958 Skype :ivy.han6 Email: [email protected] I. Configuration of JS1000 Concrete Mixer Item Unit Parameter Charging Capacity L 1600 Discharging Capacity L 1000 Min. Productivity m3 /h 50 Max Size of Aggregate (Pebble/Macadam) mm 80/60 Lifting Speed of Hopper m/min 21.9 Overall Dimension ( L x W x H) mm 8765×3436×9540 Overall Weight kg 10100 Discharge Height mm 2700/3800 Quantity 2x8 Mixing Blade Rev r/min 25.5 Type Y225S-4 Mixing Electric motor Power kw 18.5x2 Type YEZ160S-4 Windlass Electric motor Power kw 11 Type KQW65-100(I) Water Pump Electric motor Power kw 3 II. Quotation of JS1000 Concrete Mixer Name of Commodity JS1000 Concrete Mixer Quantity 1 SET Containers needed 1 SET of 40HQ FOB Qingdao Price 18,900 USD /SET Payment Terms 30%T/T in advance, 70%T/T after inspecting the finished goods before delivery Delivery 10-15 working days after receive 30% of the total amount as advance payment by T/T. Guarantee 12 months III. Remarks 1. Power source: 380V/220V, 50Hz. If power source is different, Please inform us in advance. -

Electronic Supplementary Material (ESI) for RSC Advances. This Journal Is © the Royal Society of Chemistry 2018
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2018 Supporting Information Fish scales derived carbon dots as efficient fluorescent nanoprobe for detection of ferric ions Yi Zhang,a,b Zhiyong Gao,a* Xue Yang,b Jiuli Chang,a Ziyan Liu,c and Kai Jianga,d* a School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, Henan Normal University, Henan Xinxiang 453007, P.R. China. b School of Laboratory Medicine, Xinxiang Medical University, Henan Xinxiang 453003, P.R. China. c Maternal and Child Care Service Centre of Xinxiang City, Henan Xinxiang 453000, P.R. China. d School of Environment, Henan Normal University, Henan Xinxiang 453007, P.R. China. *Corresponding authors: E-mail: [email protected] (Z.Gao) Tel./Fax: +86 373 3326336 [email protected] (K.Jiang) Tel./Fax: +86 373 3328629. Table S1 The concentrations of Fe3+ standard solutions, injected volumes and the final concentrations 3+ 3+ -1 of Fe in the CDs-Fe assay system. CCDs=250 μg mL Concentration of Fe3+ 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 standard solution (M) Volume added (μL) 0 4 8 12 16 20 24 28 32 36 40 Ultimate concentration 0 1 2 3 4 5 6 7 8 9 10 of Fe3+ in CDs-Fe3+ system (μmol L-1) Concentration of Fe3+ 0.001 0.001 0.001 0.001 0.001 0.01 0.01 0.01 0.01 0.01 0.01 standard solution (M) Volume added (μL) 44 48 64 80 96 11.2 13.2 15.2 17.2