Download Article (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drying Effects on Chemical Composition and Antioxidant Activity of Lippia Thymoides Essential Oil, a Natural Source of Thymol
molecules Article Drying Effects on Chemical Composition and Antioxidant Activity of Lippia thymoides Essential Oil, a Natural Source of Thymol Lidiane Diniz do Nascimento 1,2,* , Sebastião Gomes Silva 3 ,Márcia Moraes Cascaes 4, Kauê Santana da Costa 5,* , Pablo Luis Baia Figueiredo 6 , Cristiane Maria Leal Costa 7, Eloisa Helena de Aguiar Andrade 2,4 and Lênio José Guerreiro de Faria 1,7 1 Programa de Pós-Graduação em Engenharia de Recursos Naturais da Amazônia, Universidade Federal do Pará, Belém 66075-110, Pará, Brazil; [email protected] 2 Coordenação de Botânica, Museu Paraense Emílio Goeldi, Belém 66077-830, Pará, Brazil; [email protected] 3 Instituto de Ciências Exatas e Naturais, Universidade Federal do Pará, Belém 66075-110, Pará, Brazil; [email protected] 4 Programa de Pós-Graduação em Química, Universidade Federal do Pará, Belém 66075-110, Pará, Brazil; [email protected] 5 Faculdade de Biotecnologia, Instituto de Biodiversidade, Universidade Federal do Oeste do Pará, Santarém 68035-110, Pará, Brazil 6 Departamento de Ciências Naturais, Universidade do Estado do Pará, Belém 66050-540, Pará, Brazil; pablo.fi[email protected] 7 Programa de Pós-Graduação em Engenharia Química, Universidade Federal do Pará, Belém 66075-110, Pará, Brazil; [email protected] Citation: Nascimento, L.D.d.; Silva, * Correspondence: [email protected] (L.D.d.N.); [email protected] (K.S.d.C.); S.G.; Cascaes, M.M.; Costa, K.S.d.; Tel.: +55-91-3217-6086 (L.D.d.N.); +55-93-2101-6771 (K.S.d.C.) Figueiredo, P.L.B.; Costa, C.M.L.; Andrade, E.H.d.A.; de Faria, L.J.G. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Methylcyclopentanone Condensed with Methyl Vinyl Ketone to Give the Dione
In presenting the dissertation as a partial fulfillment of the requirements for an advanced degree from the Georgia Institute of Technology, I agree that the Library of the Institute shall make it available for inspection and circulation in accordance with its regulations governing materials of this type. I agree that permission to copy from, or to publish from, this dissertation may be granted by the professor under whose direction it was written, or, in his absence, by the Dean of the Graduate Division when such copying or publication is solely for scholarly purposes and does not involve potential f inane ia]. gain. It is under stood that any copying from, or publication of, this dis sertation which involves potential financial gain will not be allowed without written permission. 3/17/65 PART I REACTIONS OF ENOLATES DERIVED FROM UNSYMMETRICAL CYCLIC KETONES PART n PHOTOCHEMICAL REARRANGEMENTS OF CROSS CONJUGATED CYCLOHEXADIENONES RELATED TO INDAN A THESIS Presented to The Faculty of the Graduate Division by William Joseph Powers, III In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry Georgia Institute of Technology May, 1968 PART I REACTIONS OF ENOLATES DERIVED FROM UNSYMMETRICAL CYCLIC KETONES PART II PHOTOCHEMICAL REARRANGEMENTS OF CROSS CONJUGATED CYCLOHEXADIENONES RELATED TO INDAN Approved: Chairman —T, approved by Cha&limn: "JY\ 0*^ 7y 1 ii ACKNOWLEDGMENTS The author is grateful to Professor Drury S. Caine, III for suggesting these problems and for his patience and guidance throughout the course of this research. The author also wishes to thank Professors John R. Dyer and Charles L. -

Intravenous Palonosetron Increases the Incidence of Qtc Prolongation During Sevoflurane General Anesthesia for Laparotomy
Korean J Anesthesiol 2013 November 65(5): 397-402 Clinical Research Article http://dx.doi.org/10.4097/kjae.2013.65.5.397 Intravenous palonosetron increases the incidence of QTc prolongation during sevoflurane general anesthesia for laparotomy Jeong Jin Min, Yongjae Yoo, Tae Kyong Kim, and Jung-Man Lee Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea Background: Palonosetron is a recently introduced 5-hydroxytryptamine-3 (5-HT3) receptor antagonist useful for postoperative nausea and vomiting prophylaxis. However, 5-HT3 receptor antagonists increase the corrected QT (QTc) interval in patients who undergo general anesthesia. This retrospective study was performed to evaluate whether palono- setron would induce a QTc prolongation in patients undergoing general anesthesia with sevoflurane. Methods: We reviewed a database of 81 patients who underwent general anesthesia with sevoflurane. We divided the records into palonosetron (n = 41) and control (n = 40) groups according to the use of intraoperative palonosetron, and analyzed the electrocardiographic data before anesthesia and 30, 60, 90, and 120 min after skin incision. Changes in the QTc interval from baseline, mean blood pressure, heart rate, body temperature, and sevoflurane concentrations at each time point were compared between the two groups. Results: The QTc intervals at skin incision, and 30, 60, 90, and 120 min after the skin incision during general anesthesia were significantly longer than those at baseline in the two groups (P < 0.001). The changes in the QTc intervals were not different between the two groups (P = 0.41). However, six patients in the palonosetron group showed a QTc interval > 500 ms 30 min after skin incision, whereas no patient did in the control group (P = 0.01). -

Antibacterial Effect of Sevoflurane and Isoflurane Ángel Martínez-Monsalve3 María Dolores Crespo- Sánchez1
Original María Martínez-Serrano1 Manuel Gerónimo-Pardo2 Antibacterial effect of sevoflurane and isoflurane Ángel Martínez-Monsalve3 María Dolores Crespo- Sánchez1 1Servicio de Microbiología y Parasitología. Complejo Hospitalario Universitario de Albacete. 2Servicio de Anestesiología y Reanimación. Complejo Hospitalario Universitario de Albacete. 3Servicio de Cirugía Vascular. Complejo Hospitalario Universitario de Albacete. ABSTRACT property in vivo. This might then allow these agents to be con- sidered as rescue treatment against multidrug resistant patho- Introduction. Multidrug resistant bacteria are increasing gens, including a topical use in infected wounds. worldwide and therapeutic options are limited. Some anaes- Key words: Sevoflurane, Isoflurane, Anaesthetics, Inhala- thetics have shown antibacterial activity before. In this study, tion, Anti-Infective Agents. we have investigated the antibacterial effect of the halogen- ated anaesthetic agents sevoflurane and isoflurane against a range of resistant pathogens. Actividad antibacteriana de sevoflurano e Methods. Two experiments were conducted. In the first, isoflurano bacterial suspensions of both ATCC and resistant strains of Staphylococcus aureus, Escherichia coli and Pseudomonas RESUMEN aeruginosa were exposed to liquid sevoflurane and isoflurane during 15, 30 and 60 minutes. In the second experiment clinical Introducción. Las bacterias multirresistentes están au- resistant strains of E. coli, Klebsiella pneumoniae, Enterobac- mentando en todo el mundo y las opciones terapéuticas son ter cloacae, P. aeruginosa, Acinetobacter baumannii, S. aureus, limitadas. Algunos anestésicos han mostrado actividad an- and Enterococcus faecium were studied. Previously inoculated tibacteriana previamente. En este estudio hemos investigado agar plates were irrigated with the halogenated anaesthet- dicha actividad en los anestésicos halogenados sevoflurano e ic agents and these were left to evaporate before the plates isoflurano frente a un grupo de patógenos resistentes. -

Antibacterial Activity and Mechanisms of Essential Oil from Citrus Medica L
molecules Article Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis Ze-Hua Li 1,2, Ming Cai 2,*, Yuan-Shuai Liu 3, Pei-Long Sun 2,* and Shao-Lei Luo 2 1 College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310014, China; [email protected] 2 Department of Food Science and Technology, Zhejiang University of Technology, Hangzhou 310014, China; [email protected] 3 Department of Chemical and Biological Engineering, Hong Kong University of Science and Technology, Hong Kong; [email protected] * Correspondence: [email protected] (P.-L.S.); [email protected] (M.C.); Tel.: +86-0571-88320388 (P.-L.S.); +86-0571-88320345 (M.C.) Academic Editor: Francesca Mancianti Received: 29 March 2019; Accepted: 18 April 2019; Published: 22 April 2019 Abstract: In this work, antibacterial activity of finger citron essential oil (FCEO, Citrus medica L. var. sarcodactylis) and its mechanism against food-borne bacteria were evaluated. A total of 28 components in the oil were identified by gas chromatography-mass spectrometry, in which limonene (45.36%), γ-terpinene (21.23%), and dodecanoic acid (7.52%) were three main components. For in vitro antibacterial tests, FCEO exhibited moderately antibacterial activity against common food-borne bacteria: Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus. It showed a better bactericidal effect on Gram-positive bacteria than Gram-negative. Mechanisms of the antibacterial action were investigated by observing changes of bacteria morphology according to scanning electron microscopy, time-kill analysis, and permeability of cell and membrane integrity. Morphology of tested bacteria was changed and damaged more seriously with increased concentration and exposure time of FCEO. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

TRP Channel Transient Receptor Potential Channels
TRP Channel Transient receptor potential channels TRP Channel (Transient receptor potential channel) is a group of ion channels located mostly on the plasma membrane of numerous human and animal cell types. There are about 28 TRP channels that share some structural similarity to each other. These are grouped into two broad groups: Group 1 includes TRPC ("C" for canonical), TRPV ("V" for vanilloid), TRPM ("M" for melastatin), TRPN, and TRPA. In group 2, there are TRPP ("P" for polycystic) and TRPML ("ML" for mucolipin). Many of these channels mediate a variety of sensations like the sensations of pain, hotness, warmth or coldness, different kinds of tastes, pressure, and vision. TRP channels are relatively non-selectively permeable to cations, including sodium, calcium and magnesium. TRP channels are initially discovered in trp-mutant strain of the fruit fly Drosophila. Later, TRP channels are found in vertebrates where they are ubiquitously expressed in many cell types and tissues. TRP channels are important for human health as mutations in at least four TRP channels underlie disease. www.MedChemExpress.com 1 TRP Channel Antagonists, Inhibitors, Agonists, Activators & Modulators (-)-Menthol (E)-Cardamonin Cat. No.: HY-75161 ((E)-Cardamomin; (E)-Alpinetin chalcone) Cat. No.: HY-N1378 (-)-Menthol is a key component of peppermint oil (E)-Cardamonin ((E)-Cardamomin) is a novel that binds and activates transient receptor antagonist of hTRPA1 cation channel with an IC50 potential melastatin 8 (TRPM8), a of 454 nM. Ca2+-permeable nonselective cation channel, to 2+ increase [Ca ]i. Antitumor activity. Purity: ≥98.0% Purity: 99.81% Clinical Data: Launched Clinical Data: No Development Reported Size: 10 mM × 1 mL, 500 mg, 1 g Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg, 50 mg, 100 mg (Z)-Capsaicin 1,4-Cineole (Zucapsaicin; Civamide; cis-Capsaicin) Cat. -

2016 Medicines in Development for Rare Diseases a LIST of ORPHAN DRUGS in the PIPELINE
2016 Medicines in Development for Rare Diseases A LIST OF ORPHAN DRUGS IN THE PIPELINE Autoimmune Diseases Product Name Sponsor Official FDA Designation* Development Status Actemra® Genentech treatment of systemic sclerosis Phase III tocilizumab South San Francisco, CA www.gene.com Adempas® Bayer HealthCare Pharmaceuticals treatment of systemic sclerosis Phase II riociguat Whippany, NJ www.pharma.bayer.com ARA 290 Araim Pharmaceuticals treatment of neuropathic pain in patients Phase II Tarrytown, NY with sarcoidosis www.ariampharma.com ARG201 arGentis Pharmaceuticals treatment of diffuse systemic sclerosis Phase II (type 1 native bovine skin Collierville, TN www.argentisrx.com collagen) BYM338 Novartis Pharmaceuticals treatment of inclusion body myositis Phase III (bimagrumab) East Hanover, NJ www.novartis.com CCX168 ChemoCentryx treatment of anti-neutrophil cytoplasmic Phase II (5a receptor antagonist) Mountain View, CA auto-antibodies associated vasculitides www.chemocentryx.com (granulomatosis with polyangitis or Wegener's granulomatosis), microscopic polyangitis, and Churg-Strauss syndrome * This designation is issued by the FDA's Office of Orphan Products Development while the drug is still in development. The designation makes the sponsor of the drug eligible for entitlements under the Orphan Drug Act of 1983. The entitlements include seven years of marketing exclusivity following FDA approval of the drug for the designated use. Medicines in Development: Rare Diseases | 2016 1 Autoimmune Diseases Product Name Sponsor Official FDA -

Transient Receptor Potential Channels As Drug Targets: from the Science of Basic Research to the Art of Medicine
1521-0081/66/3/676–814$25.00 http://dx.doi.org/10.1124/pr.113.008268 PHARMACOLOGICAL REVIEWS Pharmacol Rev 66:676–814, July 2014 Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics ASSOCIATE EDITOR: DAVID R. SIBLEY Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine Bernd Nilius and Arpad Szallasi KU Leuven, Department of Cellular and Molecular Medicine, Laboratory of Ion Channel Research, Campus Gasthuisberg, Leuven, Belgium (B.N.); and Department of Pathology, Monmouth Medical Center, Long Branch, New Jersey (A.S.) Abstract. ....................................................................................679 I. Transient Receptor Potential Channels: A Brief Introduction . ...............................679 A. Canonical Transient Receptor Potential Subfamily . .....................................682 B. Vanilloid Transient Receptor Potential Subfamily . .....................................686 C. Melastatin Transient Receptor Potential Subfamily . .....................................696 Downloaded from D. Ankyrin Transient Receptor Potential Subfamily .........................................700 E. Mucolipin Transient Receptor Potential Subfamily . .....................................702 F. Polycystic Transient Receptor Potential Subfamily . .....................................703 II. Transient Receptor Potential Channels: Hereditary Diseases (Transient Receptor Potential Channelopathies). ......................................................704 -
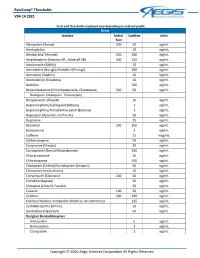
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam