Origin of Water in the Terrestrial Planets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Exoplanet Populations with NASA's Kepler Mission
SPECIAL FEATURE: PERSPECTIVE PERSPECTIVE SPECIAL FEATURE: Exploring exoplanet populations with NASA’s Kepler Mission Natalie M. Batalha1 National Aeronautics and Space Administration Ames Research Center, Moffett Field, 94035 CA Edited by Adam S. Burrows, Princeton University, Princeton, NJ, and accepted by the Editorial Board June 3, 2014 (received for review January 15, 2014) The Kepler Mission is exploring the diversity of planets and planetary systems. Its legacy will be a catalog of discoveries sufficient for computing planet occurrence rates as a function of size, orbital period, star type, and insolation flux.The mission has made significant progress toward achieving that goal. Over 3,500 transiting exoplanets have been identified from the analysis of the first 3 y of data, 100 planets of which are in the habitable zone. The catalog has a high reliability rate (85–90% averaged over the period/radius plane), which is improving as follow-up observations continue. Dynamical (e.g., velocimetry and transit timing) and statistical methods have confirmed and characterized hundreds of planets over a large range of sizes and compositions for both single- and multiple-star systems. Population studies suggest that planets abound in our galaxy and that small planets are particularly frequent. Here, I report on the progress Kepler has made measuring the prevalence of exoplanets orbiting within one astronomical unit of their host stars in support of the National Aeronautics and Space Admin- istration’s long-term goal of finding habitable environments beyond the solar system. planet detection | transit photometry Searching for evidence of life beyond Earth is the Sun would produce an 84-ppm signal Translating Kepler’s discovery catalog into one of the primary goals of science agencies lasting ∼13 h. -

Formation of TRAPPIST-1
EPSC Abstracts Vol. 11, EPSC2017-265, 2017 European Planetary Science Congress 2017 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2017 Formation of TRAPPIST-1 C.W Ormel, B. Liu and D. Schoonenberg University of Amsterdam, The Netherlands ([email protected]) Abstract start to drift by aerodynamical drag. However, this growth+drift occurs in an inside-out fashion, which We present a model for the formation of the recently- does not result in strong particle pileups needed to discovered TRAPPIST-1 planetary system. In our sce- trigger planetesimal formation by, e.g., the streaming nario planets form in the interior regions, by accre- instability [3]. (a) We propose that the H2O iceline (r 0.1 au for TRAPPIST-1) is the place where tion of mm to cm-size particles (pebbles) that drifted ice ≈ the local solids-to-gas ratio can reach 1, either by from the outer disk. This scenario has several ad- ∼ vantages: it connects to the observation that disks are condensation of the vapor [9] or by pileup of ice-free made up of pebbles, it is efficient, it explains why the (silicate) grains [2, 8]. Under these conditions plan- TRAPPIST-1 planets are Earth mass, and it provides etary embryos can form. (b) Due to type I migration, ∼ a rationale for the system’s architecture. embryos cross the iceline and enter the ice-free region. (c) There, silicate pebbles are smaller because of col- lisional fragmentation. Nevertheless, pebble accretion 1. Introduction remains efficient and growth is fast [6]. (d) At approx- TRAPPIST-1 is an M8 main-sequence star located at a imately Earth masses embryos reach their pebble iso- distance of 12 pc. -

Water, Habitability, and Detectability Steve Desch
Water, Habitability, and Detectability Steve Desch PI, “Exoplanetary Ecosystems” NExSS team School of Earth and Space Exploration, Arizona State University with Ariel Anbar, Tessa Fisher, Steven Glaser, Hilairy Hartnett, Stephen Kane, Susanne Neuer, Cayman Unterborn, Sara Walker, Misha Zolotov Astrobiology Science Strategy NAS Committee, Beckmann Center, Irvine, CA (remotely), January 17, 2018 How to look for life on (Earth-like) exoplanets: find oxygen in their atmospheres How Earth-like must an exoplanet be for this to work? Seager et al. (2013) How to look for life on (Earth-like) exoplanets: find oxygen in their atmospheres Oxygen on Earth overwhelmingly produced by photosynthesizing life, which taps Sun’s energy and yields large disequilibrium signature. Caveats: Earth had life for billions of years without O2 in its atmosphere. First photosynthesis to evolve on Earth was anoxygenic. Many ‘false positives’ recognized because O2 has abiotic sources, esp. photolysis (Luger & Barnes 2014; Harman et al. 2015; Meadows 2017). These caveats seem like exceptions to the ‘rule’ that ‘oxygen = life’. How non-Earth-like can an exoplanet be (especially with respect to water content) before oxygen is no longer a biosignature? Part 1: How much water can terrestrial planets form with? Part 2: Are Aqua Planets or Water Worlds habitable? Can we detect life on them? Part 3: How should we look for life on exoplanets? Part 1: How much water can terrestrial planets form with? Theory says: up to hundreds of oceans’ worth of water Trappist-1 system suggests hundreds of oceans, especially around M stars Many (most?) planets may be Aqua Planets or Water Worlds How much water can terrestrial planets form with? Earth- “snow line” Standard Sun distance models of distance accretion suggest abundant water. -

Title Hydrogen Atom Formation from the Photodissociation of Water Ice at 193 Nm Author(S)
Hydrogen atom formation from the photodissociation of water Title ice at 193 nm Yabushita, A; Hashikawa, Y; Ikeda, A; Kawasaki, M; Author(s) Tachikawa, H JOURNAL OF CHEMICAL PHYSICS (2004), 120(11): 5463- Citation 5468 Issue Date 2004-03-15 URL http://hdl.handle.net/2433/39757 Copyright 2004 American Institute of Physics. This article may be downloaded for personal use only. Any other use requires Right prior permission of the author and the American Institute of Physics. Type Journal Article Textversion none; publisher Kyoto University JOURNAL OF CHEMICAL PHYSICS VOLUME 120, NUMBER 11 15 MARCH 2004 Hydrogen atom formation from the photodissociation of water ice at 193 nm Akihiro Yabushita, Yuichi Hashikawa, Atsushi Ikeda, and Masahiro Kawasaki Department of Molecular Engineering, and Graduate School of Global Environmental Studies, Kyoto University, Kyoto 615-8510, Japan Hiroto Tachikawa Department of Molecular Chemistry, Graduate School of Engineering, Hokkaido University, Sapporo 060-8628, Japan ͑Received 21 April 2003; accepted 27 August 2003͒ The TOF spectra of photofragment hydrogen atoms from the 193 nm photodissociation of amorphous ice at 90–140 K have been measured. The spectra consist of both a fast and a slow ϭ Ϯ components that are characterized by average translational energies of 2kBTtrans 0.39 0.04 eV (2300Ϯ200 K) and 0.02 eV (120Ϯ20 K), respectively. The incident laser power dependency of the hydrogen atom production suggests one-photon process. The electronic excitation energy of a branched cluster, (H2O)6ϩ1 , has been theoretically calculated, where (H2O)6ϩ1 is a (H2O)6 cyclic cluster attached by a water molecule with the hydrogen bond. -

Formation of the Solar System (Chapter 8)
Formation of the Solar System (Chapter 8) Based on Chapter 8 • This material will be useful for understanding Chapters 9, 10, 11, 12, 13, and 14 on “Formation of the solar system”, “Planetary geology”, “Planetary atmospheres”, “Jovian planet systems”, “Remnants of ice and rock”, “Extrasolar planets” and “The Sun: Our Star” • Chapters 2, 3, 4, and 7 on “The orbits of the planets”, “Why does Earth go around the Sun?”, “Momentum, energy, and matter”, and “Our planetary system” will be useful for understanding this chapter Goals for Learning • Where did the solar system come from? • How did planetesimals form? • How did planets form? Patterns in the Solar System • Patterns of motion (orbits and rotations) • Two types of planets: Small, rocky inner planets and large, gas outer planets • Many small asteroids and comets whose orbits and compositions are similar • Exceptions to these patterns, such as Earth’s large moon and Uranus’s sideways tilt Help from Other Stars • Use observations of the formation of other stars to improve our theory for the formation of our solar system • Use this theory to make predictions about the formation of other planetary systems Nebular Theory of Solar System Formation • A cloud of gas, the “solar nebula”, collapses inwards under its own weight • Cloud heats up, spins faster, gets flatter (disk) as a central star forms • Gas cools and some materials condense as solid particles that collide, stick together, and grow larger Where does a cloud of gas come from? • Big Bang -> Hydrogen and Helium • First stars use this -
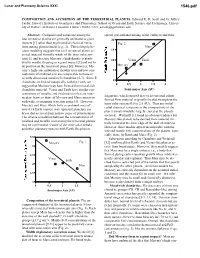
Composition and Accretion of the Terrestrial Planets
Lunar and Planetary Science XXXI 1546.pdf COMPOSITION AND ACCRETION OF THE TERRESTRIAL PLANETS. Edward R. D. Scott and G. Jeffrey Taylor, Hawai’i Institute of Geophysics and Planetology, School of Ocean and Earth Science and Technology, Univer- sity of Hawai’i at Manoa, Honolulu, Hawai’i 96822, USA; [email protected] Abstract: Compositional variations among the spread gravitational mixing of the embryos and their four terrestrial planets are generally attributed to giant 20 impacts [1] rather than to primordial chemical varia- Fig. 2 tions among planetesimals [e.g., 2]. This is largely be- Mars cause modeling suggests that each terrestrial planet ac- 15 creted material from the whole of the inner solar sys- tem [1], and because Mercury’s high density is attrib- 10 Venus Earth uted to mantle stripping in a giant impact [3] and not to its position as the innermost planet [4]. However, Mer- Mercury cury’s high concentration of metallic iron and low con- 5 centration of oxidized iron are comparable to those in recently discovered metal-rich chondrites [5-7]. Since E 0 chondrites are linked isotopically with the Earth, we 0 0.5 1 1.5 2 suggest that Mercury may have formed from metal-rich chondritic material. Venus and Earth have similar con- Semi-major Axis (AU) centrations of metallic and oxidized iron that are inter- fragments, which ensured that each terrestrial planet mediate between those of Mercury and Mars consistent formed from material originally located throughout the with wide, overlapping accretion zones [1]. However, inner solar system (0.5 to 2.5 AU). -

POSSIBLE STRUCTURE MODELS for the TRANSITING SUPER-EARTHS:KEPLER-10B and 11B
43rd Lunar and Planetary Science Conference (2012) 1290.pdf POSSIBLE STRUCTURE MODELS FOR THE TRANSITING SUPER-EARTHS:KEPLER-10b AND 11b. P. Futó1 1 Department of Physical Geography, University of West Hungary, Szombathely, Károlyi Gáspár tér, H- 9700, Hungary ([email protected]) Introduction:Up to january of 2012,10 super- The planet Kepler-11b has a large radius (1.97 R⊕) for Earths have been announced by Kepler-mission [1] its mass (4.3 M⊕),therefore this planet must have a that is designed to detect hundreds of transiting exo- spherical shell that is composed of low-density materi- planets.Kepler was launched on 6th March,2009 and the als.Considering the planet's average density,it must primary purpose of its scientific program is to search have a metallic core with different possible fractional for terrestrial-sized planets in the habitable zone of mass.Accordinghly,I have made a possible structure Solar-like stars.For the case of high number of discov- model for Kepler-11b in which the selected core mass eries we will be able to estimate the frequency of fraction is 32.59% (similarly to that of Earth) and the Earth-sized planets in our galaxy.Results of the Kepler- water ice layer has a relatively great fractional 's measurements show that the small-sized planets are volume.For case of the selected composition, the icy frequent in the spiral galaxies.A catalog of planetary surface sublimated to form a water vapor as the planet candidates,including objects with small-sized candidate moved inward the central star during its migration. -

Planetary Science
Scientific Research National Aeronautics and Space Administration Planetary Science MSFC planetary scientists are active in Peering into the history The centerpiece of the Marshall efforts is research at Marshall, building on a long history of the solar system the Marshall Noble Gas Research Laboratory of scientific support to NASA’s human explo- (MNGRL), a unique facility within NASA. Noble- ration program planning, whether focused Marshall planetary scientists use multiple anal- gas isotopes are a well-established technique on the Moon, asteroids, or Mars. Research ysis techniques to understand the formation, for providing detailed temperature-time histories areas of expertise include planetary sample modification, and age of planetary materials of rocks and meteorites. The MNGRL lab uses analysis, planetary interior modeling, and to learn about their parent planets. Sample Ar-Ar and I-Xe radioactive dating to find the planetary atmosphere observations. Scientists analysis of this type is well-aligned with the formation age of rocks and meteorites, and at Marshall are involved in several ongoing priorities for scientific research and analysis Ar/Kr/Ne cosmic-ray exposure ages to under- planetary science missions, including the Mars in NASA’s Planetary Science Division. Multiple stand when the meteorites were launched from Exploration Rovers, the Cassini mission to future missions are poised to provide new their parent planets. Saturn, and the Gravity Recovery and Interior sample-analysis opportunities. Laboratory (GRAIL) mission to the Moon. Marshall is the center for program manage- ment for the Agency’s Discovery and New Frontiers programs, providing programmatic oversight into a variety of missions to various planetary science destinations throughout the solar system, from MESSENGER’s investiga- tions of Mercury to New Frontiers’ forthcoming examination of the Pluto system. -

On the Use of Planetary Science Data for Studying Extrasolar Planets a Science Frontier White Paper Submitted to the Astronomy & Astrophysics 2020 Decadal Survey
On the Use of Planetary Science Data for Studying Extrasolar Planets A science frontier white paper submitted to the Astronomy & Astrophysics 2020 Decadal Survey Thematic Area: Planetary Systems Principal Author Daniel J. Crichton Jet Propulsion Laboratory, California Institute of Technology [email protected] 818-354-9155 Co-Authors: J. Steve Hughes, Gael Roudier, Robert West, Jeffrey Jewell, Geoffrey Bryden, Mark Swain, T. Joseph W. Lazio (Jet Propulsion Laboratory, California Institute of Technology) There is an opportunity to advance both solar system and extrasolar planetary studies that does not require the construction of new telescopes or new missions but better use and access to inter-disciplinary data sets. This approach leverages significant investment from NASA and international space agencies in exploring this solar system and using those discoveries as “ground truth” for the study of extrasolar planets. This white paper illustrates the potential, using phase curves and atmospheric modeling as specific examples. A key advance required to realize this potential is to enable seamless discovery and access within and between planetary science and astronomical data sets. Further, seamless data discovery and access also expands the availability of science, allowing researchers and students at a variety of institutions, equipped only with Internet access and a decent computer to conduct cutting-edge research. © 2019 California Institute of Technology. Government sponsorship acknowledged. Pre-decisional - For planning -

PDF— Granite-Greenstone Belts Separated by Porcupine-Destor
C G E S NT N A ER S e B EC w o TIO ok N Vol. 8, No. 10 October 1998 es st t or INSIDE Rel e • 1999 Section Meetings ea GSA TODAY Rocky Mountain, p. 25 ses North-Central, p. 27 A Publication of the Geological Society of America • Honorary Fellows, p. 8 Lithoprobe Leads to New Perspectives on 70˚ -140˚ 70˚ Continental Evolution -40˚ Ron M. Clowes, Lithoprobe, University -120˚ of British Columbia, 6339 Stores Road, -60˚ -100˚ -80˚ Vancouver, BC V6T 1Z4, Canada, 60˚ Wopmay 60˚ [email protected] Slave SNORCLE Fred A. Cook, Department of Geology & Thelon Rae Geophysics, University of Calgary, Calgary, Nain Province AB T2N 1N4, Canada 50˚ ECSOOT John N. Ludden, Centre de Recherches Hearne Pétrographiques et Géochimiques, Taltson Vandoeuvre-les-Nancy, Cedex, France AB Trans-Hudson Orogen SC THOT LE WS Superior Province ABSTRACT Cordillera AG Lithoprobe, Canada’s national earth KSZ o MRS 40 40 science research project, was established o Grenville Province in 1984 to develop a comprehensive Wyoming Penokean GL -60˚ understanding of the evolution of the -120˚ Yavapai Province Orogen Appalachians northern North American continent. With rocks representing 4 b.y. of Earth -100˚ -80˚ history, the Canadian landmass and off- Phanerozoic Proterozoic Archean shore margins provide an exceptional 200 Ma - present 1100 Ma 3200 - 2650 Ma opportunity to gain new perspectives on continental evolution. Lithoprobe’s 470 - 275 Ma 1300 - 1000 Ma 3400 - 2600 Ma 10 study areas span the country and 1800 - 1600 Ma 3800 - 2800 Ma geological time. A pan-Lithoprobe syn- 1900 - 1800 Ma 4000 - 2500 Ma thesis will bring the project to a formal conclusion in 2003. -
![Arxiv:1106.3917V2 [Astro-Ph.IM] 9 Feb 2015](https://docslib.b-cdn.net/cover/3549/arxiv-1106-3917v2-astro-ph-im-9-feb-2015-363549.webp)
Arxiv:1106.3917V2 [Astro-Ph.IM] 9 Feb 2015
van Dishoeck & Visser: Molecular Photodissociation 1 published as a chapter in Laboratory Astrochemistry From Molecules through Nanoparticles to Grains eds. S. Schlemmer, T. Giesen, H. Mutschke, and C. Jäger 2015, Weinheim: Wiley-VCH 4 Molecular photodissociation Ewine F. van Dishoeck1;2 & Ruud Visser3;4 1Leiden Observatory, Leiden University, P.O. Box 9513, 2300 RA Leiden, the Netherlands 2Max-Planck-Institut für Extraterrestrische Physik, Giessenbachstrasse 1, 85748 Garching, Germany 3Department of Astronomy, University of Michigan, 1085 S. University Ave, Ann Arbor, MI 48109-1107, USA 4European Southern Observatory, Karl-Schwarzschild-Str. 2, 85748, Garching, Germany 4.1 Introduction Photodissociation is the dominant process by which molecules are removed in any region exposed to intense ultraviolet (UV) radiation. Such clouds of gas and dust are indicated by astronomers with the generic title ‘photon-dominated region’, or PDR. Originally, the term PDR referred mostly to dense molecular clouds close to bright young stars such as found in the Orion nebula. There are many other regions in space, however, in which photodissociation plays a crucial role in the chemistry: this includes diffuse and translucent interstellar clouds, high velocity shocks, the surface layers of protoplanetary disks, and cometary and exoplanetary atmospheres. In the simplest case, a molecule ABC absorbs a UV photon, which promotes it in- to an excited electronic state, and subsequently dissociates to AB + C. In reality, the arXiv:1106.3917v2 [astro-ph.IM] 9 Feb 2015 situation is much more complex because there are many electronic states that can be excited, with only a fraction of the absorptions leading to dissociation. Also, there are many possible photodissociation products, each of which can be produced in dif- ferent electronic, vibrational and rotational states depending on the wavelength of the incident photons. -

In Vivo H NMR Methods to Study Dynamics of Chloroplast Water and Thylakoid Membrane Lipids in Leaves and in Photosynthetic Microorganisms
In vivo 1H NMR methods to study dynamics of chloroplast water and thylakoid membrane lipids in leaves and in photosynthetic microorganisms Shanthadevi Pagadala Thesis committee Promotor Prof. Dr H. Van Amerongen Professor of Biophysics Wageningen University & Research Co-promotor Dr H. Van As Associate professor, Laboratory of Biophysics Wageningen University & Research Other members Prof. Dr L. F. M. Marcelis, Wageningen University & Research Prof. Dr J. A. Killian, Utrecht University, The Netherlands Dr C. P. M. Van Mierlo, Wageningen University & Research Dr A. Pandit, Leiden University, The Netherlands This research was conducted under the auspices of the Graduate School Experimental Plant Sciences. 1 In vivo H NMR methods to study dynamics of chloroplast water and thylakoid membrane lipids in leaves and in photosynthetic microorganisms Shanthadevi Pagadala Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr. A. P. J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Tuesday 6 June 2017 at 11.00 a.m. in the Aula. Shanthadevi Pagadala In vivo 1H NMR methods to study dynamics of chloroplast water and thylakoid membrane lipids in leaves and in photosynthetic microorganisms 130 pages. PhD thesis, Wageningen University, Wageningen, NL (2017) With references, with summary in English ISBN: 978-94-6343-156-9 DOI: 10.18174/411095 Contents 1. Introduction 1 2. Chloroplast water in leaves as viewed by 1H DOSY and 13 DRCOSY NMR 3. The effect of dehydration on leaf cellular compartments 45 in relation to the water buffering role of subepidermal cells studied by 1H DOSY 4.