Guidelines for Intravenous Albumin Administration at Stanford Health Care
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopy As a Diagnostic Tool in Ascites of Unknown Origin: a Retrospective Study Conducted at Kasturba Hospital, Manipal
Research Article Open Access J Surg Volume 8 Issue 3 - March 2018 Copyright © All rights are reserved by Chetan R Kulkarni DOI: 10.19080/OAJS.2018.08.555740 Laparoscopy as a Diagnostic Tool in Ascites of Unknown Origin: A Retrospective Study Conducted at Kasturba Hospital, Manipal Chetan R Kulkarni1*, Badareesh Laxminarayan2 and Annappa Kudva3 1Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal 2Associate Professor, Department of Surgery, Kasturba Hospital, Manipal 3Professor, Department of Surgery, Kasturba Hospital, Manipal Received: February 16, 2018; Published: March 01, 2018 *Corresponding author: Chetan R Kulkarni, Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal, India. Tel: 9535974395;Email: Abstract Background: Laparoscopy as a minimally invasive technique has long played an important role in the evaluation of ascites. Methods: A retrospective analysis was carried out on the record of 80patients who underwent laparoscopy after appropriate investigations had failed to reveal the cause of ascites. Results: Tuberculous peritonitis was reported in 46(57%), malignancies in 18(25%), cirrhosis in 4(5%) and peritonitis of unknown etiology in 8(10%)Conclusion: of patients. Two (2.5%) patients had complications, an Ileal perforation and in other Incisional hernia. Keywords: Ascites;Laparoscopy Diagnostic was Laparoscopy able to diagnose the pathology in 72 (90%) patients with ascites of unknown origin. Introduction Laparoscopy, as a minimally invasive technique has developed rapidly in recent years. Endoscopic examination of conventional laboratory examinations (including ascitic fluid cell count, albumin level, total protein level, Gram stain, culture who termed it as “Celioscopy” [1,2]. The term ‘ascites’ refers to and cytology ) as well as after imaging investigations (including peritoneal cavity was first attempted in 1901 by George Kelling Materialultrasound andand CT Methods scan). -

AAT) After Portal Vein Injection of Recombinant Adeno-Associated Virus (Raav) Vectors
Gene Therapy (2001) 8, 1299–1306 2001 Nature Publishing Group All rights reserved 0969-7128/01 $15.00 www.nature.com/gt RESEARCH ARTICLE Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors S Song1,4, J Embury2,4, PJ Laipis2,4, KI Berns1,4, JM Crawford2 and TR Flotte1,4 Departments of 1Pediatrics, 2Biochemistry and Molecular Biology, and 3Pathology, and 4Powell Gene Therapy Centre of the University of Florida Genetics Institute, University of Florida, Gainesville, FL, USA Previous work from our group showed that recombinant after portal vein injection of doses of 4 × 109 infectious units adeno-associated virus (rAAV) vectors mediated long-term (IU), a 10-fold lower dose than that required for similar levels secretion of therapeutic serum levels of human alpha-1 anti- of expression via the i.m. route. Serum levels greater than trypsin (hAAT) after a single injection in murine muscle. We 1 mg/ml were achieved at doses of 3 × 1010 IU. Southern hypothesized that hepatocyte transduction could be even blotting of liver DNA revealed the presence of circular episo- more efficient, since these cells represent the natural site of mal vector genomes. Immunostaining showed that trans- AAT production and secretion. To test this hypothesis, rAAV gene expression was scattered throughout the liver paren- vectors containing the hAAT cDNA driven by either the chyma. Similar results were obtained with a rAAV-CB-green human elongation factor 1 alpha promoter, the human cyto- fluorescent protein (GFP) vector. There was no evidence of megalovirus immediate–early promoter (CMV), or the CMV- hepatic toxicity. -

Alpha1-Antitrypsin, a Reliable Endogenous Marker for Intestinal Protein Loss and Its Application in Patients with Crohn's Disease
Gut: first published as 10.1136/gut.24.8.718 on 1 August 1983. Downloaded from Gut, 1983, 24, 718-723 Alpha1-antitrypsin, a reliable endogenous marker for intestinal protein loss and its application in patients with Crohn's disease U KARBACH, K EWE, AND H BODENSTEIN From the I. Medizinische Klinik und Poliklinik, Mainz, FR Germany. SUMMARY Intestinal protein loss is generally determined by radio-labelled macromolecules. Alpha1-antitrypsin has been proposed as an endogenous marker for protein losing enteropathy, but different opinions exist about its reliability. In 25 patients with Crohn's disease faecal protein loss was studied with intestinal alpha1-antitrypsin (x1AT) clearance. Simultaneously, in 10 patients x1AT clearance was compared with faecal 51Cr clearance after intravenous 51Cr-albumin injection. There was a linear relation (p<0O05) between X1AT clearance and 51Cr clearance in these cases. In all patients ox1AT clearance was raised above control values. &1AT clearance, however, did not correlate with the activity index of Crohn's disease.1 This index does not contain direct critieria of intestinal inflammation, does not take into account localisation or extent of inflammation, and includes complications such as extraintestinal manifestations, fistuli, stenoses not necessarily related to actual mucosal involvement. It is concluded that x1AT is a reliable marker for intestinal protein loss and that the intestinal changes of Crohn's disease generally lead to an increased protein exudation into the gut. http://gut.bmj.com/ Gastrointestinal loss of plasma proteins can be clearance with the conventional 5 Cr-albumin detected by a variety of labelled macromolecules: method. According to Keaney and Kelleherl° the 59Fe-labelled dextran and 131I_PVP3 are not split by contradictory results could be caused by a difference digestive enzymes; the radioactive isotopes 51Cr- in methods and by comparison of different albumin,4 67Cu-ceruloplasmins or 95Nb-albumin6 are parameters. -

Recurrent Pneumothorax Following Abdominal Paracentesis
Postgrad Med J (1990) 66, 319 - 320 © The Fellowship of Postgraduate Medicine, 1990 Postgrad Med J: first published as 10.1136/pgmj.66.774.319 on 1 April 1990. Downloaded from Recurrent pneumothorax following abdominal paracentesis P.J. Stafford Department ofMedicine, Newham General Hospital, Glen Road, Plaistow, London E13, UK. Summary: A 62 year old man presented with abdominal ascites, without pleural effusion, due to peritoneal mesothelioma. He had chronic obstructive airways disease and a past history of right upper lobectomy for tuberculosis. On two occasions abdominal paracentesis was followed within 72 hours by pneumothorax. This previously unreported complication of abdominal paracentesis may be due to increased diaphragmatic excursion following the procedure and should be considered in patients with preexisting lung disease. Introduction Abdominal paracentesis is a widely used palliative right iliac fossa. Approximately 4 litres of turbid therapy for malignant ascites and is generally fluid was drained over 72 hours. The protein accepted as a procedure with few adverse effects: concentration was 46 g/l and microbiology and pneumothorax is not a recognized complication. I cytology were not diagnostic. Laparoscopy re- report a patient with recurrent pneumothorax vealed matted loops of bowel with widespread apparently precipitated by abdominal paracen- peritoneal seedlings. Histological examination of copyright. tesis. these lesions showed malignant mesothelioma of the epithelioid type. The patient suffered a left pneumothorax within Case report 48 hours ofparacentesis (before laparoscopy). This was treated with intercostal underwater drainage A 62 year old civil servant presented with a 6-week but recurred on two attempts to remove the drain, history ofworsening abdominal distension. -

The Importance of Early Identification of Alpha-1 Antitrypsin Deficiency
Open Access Case Report DOI: 10.7759/cureus.3494 The Importance of Early Identification of Alpha-1 Antitrypsin Deficiency Barjinder S. Buttar 1 , Mark Bernstein 1 1. Internal Medicine, Zucker School of Medicine / Northwell Health Mather Hospital, Port Jefferson, USA Corresponding author: Barjinder S. Buttar, [email protected] Abstract Alpha-1 antitrypsin deficiency (AATD) is a common genetic disorder that is easily managed if diagnosed and treated at an early age. It is often missed, however, especially in patients with long histories of smoking and alcohol use. This is mainly due to a lack of awareness and proper screening of the disorder, especially in the primary care setting. Here, we will focus on a case report of a young male whose diagnosis and treatment of AATD was significantly delayed. His lung and liver complications had initially been attributed to his smoking and drinking history. This delay could have been avoided by increasing awareness of AATD and through the implementation of novel screening tests that can quickly rule out the disorder in patients presenting with lung and liver disease. Categories: Internal Medicine, Preventive Medicine, Rheumatology Keywords: aatd, emphysema, copd, cirrhosis, prolastin, screening, alpha-1 antitrypsin deficiency Introduction Alpha-1 antitrypsin deficiency (AATD) is a common autosomal recessive disorder. Alpha-1 antitrypsin (AAT) is defined as a protease inhibitor which is encoded by the SERPINA1 gene. M refers to the normal allele while Z refers to the mutated allele. The mutated Z allele is carried by approximately 2 - 3% of the Caucasian population in the United States. Homozygosity of the Z allele, PI*ZZ, is the most common mutation that leads to AATD [1]. -
Intestinal and Multiple Organ Transplantation 1679
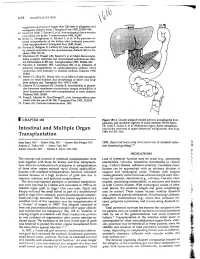
1678 TRANSPLANT ATION euglycemia and survive longer than 200 islets in allogeneic and xenogeneic diabetic hosts. Transplant Proc 1993; 25:953-954. 67. Gotoh M, Maki T, Satomi S, et al: Immunological characteristics of purified islet grafts. Transplantation 1986; 42j387. 68. Klima G, Konigsrainer A, Schmid T, et al: Is the pancreas reo jected independently of the kidney after combined pancreatic renal transplantation? Transplant Proc 1988; 20:665. 69. Prowse 5J, Bellgrau D, Lafferty KJ: Islet allografts are destroyed by disease recurrence in the spontaneously diabetic BB rat. Di abetes 1986; 35:110. 70. Markmann JF, Posselt AM, Bassiri H, et al: Major-histocompat ibility-complex restricted and nonrestricted autoil1'\mune effec tor mechanisms in BB rats. Transplantation 1991; 52:662-667. 71. Navarro X, Kennedy WR, Loewenson RB, et al: Influence of pancreas transplantation on cardiorespiratory reflexes, nerve conduction, and mortality in diabetes mellitus. Diabetes 1990; 39:802. 72. Weber q, Silva FG, Hardy MA, et al: Effect of islet transplan tation on renal function and morphology of short- and long term diabetic rats. Transplant Proc 1979; 11:549. 73. Gotzche 0, Gunderson HJ, Osterby R: Irreversibility of glomer ular basement membrane accumulation despite reversibility of renal hypertrophy with islet transplantation in early diabetes. Diabetes 1981; 30:481. 74. Fung H, Alessini M, Abu-Elmagd K, et al: Adverse effects asso ciated with the use ofFK 506. Transplant Proc 1991; 23:3105. 75. Tzakis AG: Personal communication, 1991. I CHAPTER 185 Figure 185-1. Cluster allograft (shaded portion), including the liver, pancreas, and duodenal segment of small intestine. (From Starzl TE, Todo S. -

Guidelines on the Management of Ascites in Cirrhosis
Downloaded from gut.bmjjournals.com on 25 September 2006 Guidelines on the management of ascites in cirrhosis K P Moore and G P Aithal Gut 2006;55;1-12 doi:10.1136/gut.2006.099580 Updated information and services can be found at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1 These include: References This article cites 148 articles, 21 of which can be accessed free at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1#BIBL Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Topic collections Articles on similar topics can be found in the following collections Liver, including hepatitis (945 articles) Notes To order reprints of this article go to: http://www.bmjjournals.com/cgi/reprintform To subscribe to Gut go to: http://www.bmjjournals.com/subscriptions/ Downloaded from gut.bmjjournals.com on 25 September 2006 vi1 GUIDELINES Guidelines on the management of ascites in cirrhosis K P Moore, G P Aithal ............................................................................................................................... Gut 2006;55(Suppl VI):vi1–vi12. doi: 10.1136/gut.2006.099580 1.0 INTRODUCTION N Grade 1 (mild). Ascites is only detectable by ultrasound examination. Ascites is a major complication of cirrhosis,1 occurring in 50% of patients over 10 years of N Grade 2 (moderate). Ascites causing moderate follow up.2 The development of ascites is an symmetrical distension of the abdomen. important landmark in the natural history of N Grade 3 (large). Ascites causing marked cirrhosis as it is associated with a 50% mortality abdominal distension. -

Procedure Coding in ICD-9-CM and ICD- 10-PCS
Procedure Coding in ICD-9-CM and ICD- 10-PCS ICD-9-CM Volume 3 Procedures are classified in volume 3 of ICD-9-CM, and this section includes both an Alphabetic Index and a Tabular List. This volume follows the same format, organization and conventions as the classification of diseases in volumes 1 and 2. ICD-10-PCS ICD-10-PCS will replace volume 3 of ICD-9-CM. Unlike ICD-10-CM for diagnoses, which is similar in structure and format as the ICD-9-CM volumes 1 and 2, ICD-10-PCS is a completely different system. ICD-10-PCS has a multiaxial seven-character alphanumeric code structure providing unique codes for procedures. The table below gives a brief side-by-side comparison of ICD-9-CM and ICD-10-PCS. ICD-9-CM Volume3 ICD-10-PCS Follows ICD structure (designed for diagnosis Designed and developed to meet healthcare coding) needs for a procedure code system Codes available as a fixed or finite set in list form Codes constructed from flexible code components (values) using tables Codes are numeric Codes are alphanumeric Codes are 3-4 digits long All codes are seven characters long ICD-9-CM and ICD-10-PCS are used to code only hospital inpatient procedures. Hospital outpatient departments, other ambulatory facilities, and physician practices are required to use CPT and HCPCS to report procedures. ICD-9-CM Conventions in Volume 3 Code Also In volume 3, the phrase “code also” is a reminder to code additional procedures only when they have actually been performed. -

VECTASTAIN® ABC Kit Reagents Should Be Stored at 2-8 °C
COMPONENTS STAINING PROCEDURE Reagents supplied: 1. For paraffin sections, deparaffinize and hydrate through xylenes or other clearing agents and graded alcohol series. • Blocking Serum (Normal Serum) in yellow-labeled small bottle – 3 ml For frozen sections or cell preparations fix with acetone or an appropriate • Biotinylated, Affinity-purified Anti-Immunoglobulin in blue-labeled small fixative for the antigen under study, if necessary. bottle – 1 ml Wash for 5 minutes in tap water. • Reagent A (Avidin DH) in orange-labeled small bottle – 2 ml 2. If antigen unmasking is required, perform this procedure using a Vector® • Reagent B (Biotinylated Horseradish Peroxidase H) in brown-labeled small Antigen Unmasking Solution, Citrate-based, pH 6.0 (H-3300) or Tris-based, pH bottle – 2 ml 9.0 (H-3301). ® The VECTASTAIN ABC Kit contains sufficient reagents to stain approximately 1000- 3. If quenching of endogenous peroxidase activity is required, incubate the 2000 tissue sections. slides in BLOXALL™ Blocking Solution (SP-6000) for 10 minutes. If endogenous peroxidase activity does not present a problem, this step may be omitted. For ® NOTE: The VECTASTAIN ABC Kit (Standard), Cat. No. PK-4000, contains only alternative quenching procedures please see Note 3. Reagent A and Reagent B. ® 4. Wash in buffer for 5 minutes. VECTASTAIN ABC KIT Storage: Stock VECTASTAIN® ABC Kit reagents should be stored at 2-8 °C. 5. Incubate for 20 minutes with diluted normal blocking serum. (In cases where non-specific staining is not a problem, steps 5 and 6 can be omitted).* Reagents not supplied: INSTRUCTIONS FOR 6. Blot excess serum from sections. -

Monoclonal Anti-Bovine Serum Albumin Antibody
Product No. B-2901 Lot 027H4822 Monoclonal Anti-Bovine Serum Albumin (BSA) Mouse Ascites Fluid Clone BSA-33 Monoclonal Anti-Bovine Serum Albumin (BSA) Description (mouse IgG2a isotype) is produced by the fusion of mouse myeloma cells and splenocytes from an immu- Bovine serum albumin is the major protein produced by nized mouse. Bovine serum albumin was used as the the liver and represents more than half of the total immunogen. The isotype is determined using Sigma protein found in serum. BSA is found in many biologi- ImmunoTypeTM Kit (Sigma Stock No. ISO-1) and by a cal substances such as serum supplemented cell culture double diffusion immunoassay using Mouse Mono- media and its products, in foods and forensic prepara- clonal Antibody Isotyping Reagents (Sigma Stock No. tions. A monoclonal antibody of species specificity ISO-2). The product is provided as a liquid with 0.1% may prove useful in the identification of bovine serum sodium azide (see MSDS)* as a preservative. albumin. Specificity Uses Monoclonal Anti-BSA recognizes the 67 kD band of Monoclonal Anti-Bovine Serum Albumin may be used SDS-denatured and reduced BSA using an immunoblot- for determination and quantification of BSA by ELISA, ting technique. The antibody is specific for bovine competitive ELISA and immunodot blot. The antibody serum albumin and is highly cross reactive with goat may be used for the immunoaffinity purification and and sheep serum albumins. The product is somewhat removal of BSA from various biological fluids such as less cross reactive with dog, turkey and horse serum cell culture media and in vitro-produced monoclonal albumins. -

AASLD Position Paper : Liver Biopsy
AASLD POSITION PAPER Liver Biopsy Don C. Rockey,1 Stephen H. Caldwell,2 Zachary D. Goodman,3 Rendon C. Nelson,4 and Alastair D. Smith5 This position paper has been approved by the AASLD and College of Cardiology and the American Heart Associa- represents the position of the association. tion Practice Guidelines3).4 Introduction Preamble Histological assessment of the liver, and thus, liver bi- These recommendations provide a data-supported ap- opsy, is a cornerstone in the evaluation and management proach. They are based on the following: (1) formal re- of patients with liver disease and has long been considered view and analysis of the recently published world to be an integral component of the clinician’s diagnostic literature on the topic; (2) American College of Physi- armamentarium. Although sensitive and relatively accu- cians Manual for Assessing Health Practices and De- rate blood tests used to detect and diagnose liver disease signing Practice Guidelines1; (3) guideline policies, have now become widely available, it is likely that liver including the AASLD Policy on the Development and biopsy will remain a valuable diagnostic tool. Although Use of Practice Guidelines and the American Gastro- histological evaluation of the liver has become important enterological Association Policy Statement on Guide- in assessing prognosis and in tailoring treatment, nonin- lines2; and (4) the experience of the authors in the vasive techniques (i.e., imaging, blood tests) may replace specified topic. use of liver histology in this setting, particularly with re- Intended for use by physicians, these recommenda- gard to assessment of the severity of liver fibrosis.5,6 Sev- tions suggest preferred approaches to the diagnostic, ther- eral techniques may be used to obtain liver tissue; a table apeutic, and preventive aspects of care. -

Biochemical Characterization of Multiple Myeloma Patients Across ISS Stages – a Data
apjcc.waocp.com Noorjahan Mohammed, et al: Biochemical Characterization of Multiple Myeloma Patients across ISS Stages – A Data DOI:10.31557/APJCC.2019.4.3.77 RESEARCH ARTICLE Biochemical Characterization of Multiple Myeloma Patients across ISS Stages – A Data Base Workup from a Tertiary Care Hospital in India Noorjahan Mohammed1, KSS SaiBaba1, Yadagiri.B1, Sadasivudu Gundeti2, Sree Bhushan Raju3 1Department of Biochemistry, Hyderabad, Telangana, India 500082. 2Department of Medical Oncology, Hyderabad, Telangana, India 500082. 3Department of Nephrology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India 500082. Abstract Background: Multiple myeloma (MM) is slowly becoming a huge medical burden, challenging the health-care systems of Asian countries. Because of the unavailability of widespread access to various modalities of investigations, and paucity of well compiled data on common presenting features and various laboratory parameters in various stages of MM in India, the diagnosis is usually delayed till complications begin to occur. This study is an attempt to fill this gap and to establish database for future reference. Methods: The study was conducted in a tertiary health care centre over a span of 3 years and 94 patients diagnosed as MM with complete workup including beta2 microglobulin (β2M), bone marrow plasma cell percentage, serum protein electrophoresis, serum and urine Immunofixation and serum Free Light Chains (FLC) were included. The various laboratory parameters were statistically analyzed across ISS stages I, II and III. Results: We found a male to female ratio of 1.47:1. The mean age of patients was 55.5±11.78 yrs. Backache was the most frequent presentation (30%) of the patients followed by generalized weakness (22%).