5. Guidelines for the Management of Malignant Ascites in Palliative Care
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopy As a Diagnostic Tool in Ascites of Unknown Origin: a Retrospective Study Conducted at Kasturba Hospital, Manipal
Research Article Open Access J Surg Volume 8 Issue 3 - March 2018 Copyright © All rights are reserved by Chetan R Kulkarni DOI: 10.19080/OAJS.2018.08.555740 Laparoscopy as a Diagnostic Tool in Ascites of Unknown Origin: A Retrospective Study Conducted at Kasturba Hospital, Manipal Chetan R Kulkarni1*, Badareesh Laxminarayan2 and Annappa Kudva3 1Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal 2Associate Professor, Department of Surgery, Kasturba Hospital, Manipal 3Professor, Department of Surgery, Kasturba Hospital, Manipal Received: February 16, 2018; Published: March 01, 2018 *Corresponding author: Chetan R Kulkarni, Assistant Professor, Department of Surgery, Kasturba Hospital, Manipal, India. Tel: 9535974395;Email: Abstract Background: Laparoscopy as a minimally invasive technique has long played an important role in the evaluation of ascites. Methods: A retrospective analysis was carried out on the record of 80patients who underwent laparoscopy after appropriate investigations had failed to reveal the cause of ascites. Results: Tuberculous peritonitis was reported in 46(57%), malignancies in 18(25%), cirrhosis in 4(5%) and peritonitis of unknown etiology in 8(10%)Conclusion: of patients. Two (2.5%) patients had complications, an Ileal perforation and in other Incisional hernia. Keywords: Ascites;Laparoscopy Diagnostic was Laparoscopy able to diagnose the pathology in 72 (90%) patients with ascites of unknown origin. Introduction Laparoscopy, as a minimally invasive technique has developed rapidly in recent years. Endoscopic examination of conventional laboratory examinations (including ascitic fluid cell count, albumin level, total protein level, Gram stain, culture who termed it as “Celioscopy” [1,2]. The term ‘ascites’ refers to and cytology ) as well as after imaging investigations (including peritoneal cavity was first attempted in 1901 by George Kelling Materialultrasound andand CT Methods scan). -

Recurrent Pneumothorax Following Abdominal Paracentesis
Postgrad Med J (1990) 66, 319 - 320 © The Fellowship of Postgraduate Medicine, 1990 Postgrad Med J: first published as 10.1136/pgmj.66.774.319 on 1 April 1990. Downloaded from Recurrent pneumothorax following abdominal paracentesis P.J. Stafford Department ofMedicine, Newham General Hospital, Glen Road, Plaistow, London E13, UK. Summary: A 62 year old man presented with abdominal ascites, without pleural effusion, due to peritoneal mesothelioma. He had chronic obstructive airways disease and a past history of right upper lobectomy for tuberculosis. On two occasions abdominal paracentesis was followed within 72 hours by pneumothorax. This previously unreported complication of abdominal paracentesis may be due to increased diaphragmatic excursion following the procedure and should be considered in patients with preexisting lung disease. Introduction Abdominal paracentesis is a widely used palliative right iliac fossa. Approximately 4 litres of turbid therapy for malignant ascites and is generally fluid was drained over 72 hours. The protein accepted as a procedure with few adverse effects: concentration was 46 g/l and microbiology and pneumothorax is not a recognized complication. I cytology were not diagnostic. Laparoscopy re- report a patient with recurrent pneumothorax vealed matted loops of bowel with widespread apparently precipitated by abdominal paracen- peritoneal seedlings. Histological examination of copyright. tesis. these lesions showed malignant mesothelioma of the epithelioid type. The patient suffered a left pneumothorax within Case report 48 hours ofparacentesis (before laparoscopy). This was treated with intercostal underwater drainage A 62 year old civil servant presented with a 6-week but recurred on two attempts to remove the drain, history ofworsening abdominal distension. -

Atypical Abdominal Pain in a Patient with Liver Cirrhosis
IMAGE IN HEPATOLOGY January-February, Vol. 17 No. 1, 2018: 162-164 The Official Journal of the Mexican Association of Hepatology, the Latin-American Association for Study of the Liver and the Canadian Association for the Study of the Liver Atypical Abdominal Pain in a Patient With Liver Cirrhosis Liz Toapanta-Yanchapaxi,* Eid-Lidt Guering,** Ignacio García-Juárez* * Gastroenterology Department. National Institute of Medical Science and Nutrition “Salvador Zubirán”. Mexico City. Mexico. ** Interventional Cardiology. National Institute of Cardiology “Ignacio Chávez”. Mexico City. Mexico. ABSTRACTABSTABSTABSTRACT The causes of abdominal pain in patients with liver cirrhosis and ascites are well-known but occasionally, atypical causes arise. We report the case of a patient with a ruptured, confined abdominal aortic aneurysm. KeyK words.o d .K .Key Liver cirrhosis. Abdominal aortic aneurysm. INTRODUCTION CASE REPORT Abdominal pain in cirrhotic patients is a challenge. A 47-year-old male with the established diagnosis of Clinical presentation can be non-specific and the need for alcohol-related liver cirrhosis, presented to the emer- early surgical exploration may be difficult to assess. Coag- gency department for abdominal pain. He was classified ulopathy, thrombocytopenia, varices, and ascites need to as Child Pugh (CP) C stage (10 points) and had a model be taken into account, since they can increase the surgical for end stage liver disease (MELD) - sodium (Na) of 21 risk. Possible differential diagnoses include: Complicated points. The pain was referred to the left iliac fossa, with umbilical, inguinal or postoperative incisional hernias, an intensity of 10/10. It was associated to low back pain acute cholecystitis, spontaneous bacterial peritonitis, pep- with radicular stigmata (primarily S1). -

High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider
NUTRITIONINFLAMMATORY ISSUES BOWEL IN GASTROENTEROLOGY, DISEASE: A PRACTICAL SERIES APPROACH, #105 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider Iris Vance Neeral Shah Percutaneous endoscopy gastrostomy (PEG) tubes are a valuable tool for providing long- term enteral nutrition or gastric decompression; certain circumstances that complicate PEG placement warrant novel approaches and merit review and discussion. Ascites and portal hypertension with varices have been associated with poorer outcomes. Bleeding is one of the most common serious complications affecting approximately 2.5% of all procedures. This article will review what evidence exists in these high risk scenarios and attempt to provide more clarity when considering these challenging clinical circumstances. INTRODUCTION ince the first Percutaneous Endoscopic has been found by multiple authors to portend a poor Gastrostomy tube was placed in 1979 (1), they prognosis in PEG placement (3,4, 5,6,7,8). This review Shave become an invaluable tool for providing will endeavor to provide more clarity when considering long-term enteral nutrition (EN) and are commonly used these challenging clinical circumstances. in patients with dysphagia following stroke, disabling motor neuron diseases such as multiple sclerosis and Ascites & Gastric Varices amyotrophic lateral sclerosis, and in those with head The presence of ascites is frequently viewed as a and neck cancer.They are also used for patients with relative, if not absolute, contraindication to PEG prolonged mechanical intubation, as well as gastric placement. Ascites adds technical difficulties and the decompression in those with severe gastroparesis, risk for potential complications (see Table 1). -
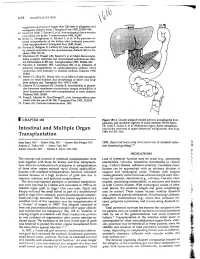
Intestinal and Multiple Organ Transplantation 1679
1678 TRANSPLANT ATION euglycemia and survive longer than 200 islets in allogeneic and xenogeneic diabetic hosts. Transplant Proc 1993; 25:953-954. 67. Gotoh M, Maki T, Satomi S, et al: Immunological characteristics of purified islet grafts. Transplantation 1986; 42j387. 68. Klima G, Konigsrainer A, Schmid T, et al: Is the pancreas reo jected independently of the kidney after combined pancreatic renal transplantation? Transplant Proc 1988; 20:665. 69. Prowse 5J, Bellgrau D, Lafferty KJ: Islet allografts are destroyed by disease recurrence in the spontaneously diabetic BB rat. Di abetes 1986; 35:110. 70. Markmann JF, Posselt AM, Bassiri H, et al: Major-histocompat ibility-complex restricted and nonrestricted autoil1'\mune effec tor mechanisms in BB rats. Transplantation 1991; 52:662-667. 71. Navarro X, Kennedy WR, Loewenson RB, et al: Influence of pancreas transplantation on cardiorespiratory reflexes, nerve conduction, and mortality in diabetes mellitus. Diabetes 1990; 39:802. 72. Weber q, Silva FG, Hardy MA, et al: Effect of islet transplan tation on renal function and morphology of short- and long term diabetic rats. Transplant Proc 1979; 11:549. 73. Gotzche 0, Gunderson HJ, Osterby R: Irreversibility of glomer ular basement membrane accumulation despite reversibility of renal hypertrophy with islet transplantation in early diabetes. Diabetes 1981; 30:481. 74. Fung H, Alessini M, Abu-Elmagd K, et al: Adverse effects asso ciated with the use ofFK 506. Transplant Proc 1991; 23:3105. 75. Tzakis AG: Personal communication, 1991. I CHAPTER 185 Figure 185-1. Cluster allograft (shaded portion), including the liver, pancreas, and duodenal segment of small intestine. (From Starzl TE, Todo S. -

Guidelines on the Management of Ascites in Cirrhosis
Downloaded from gut.bmjjournals.com on 25 September 2006 Guidelines on the management of ascites in cirrhosis K P Moore and G P Aithal Gut 2006;55;1-12 doi:10.1136/gut.2006.099580 Updated information and services can be found at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1 These include: References This article cites 148 articles, 21 of which can be accessed free at: http://gut.bmjjournals.com/cgi/content/full/55/suppl_6/vi1#BIBL Email alerting Receive free email alerts when new articles cite this article - sign up in the box at the service top right corner of the article Topic collections Articles on similar topics can be found in the following collections Liver, including hepatitis (945 articles) Notes To order reprints of this article go to: http://www.bmjjournals.com/cgi/reprintform To subscribe to Gut go to: http://www.bmjjournals.com/subscriptions/ Downloaded from gut.bmjjournals.com on 25 September 2006 vi1 GUIDELINES Guidelines on the management of ascites in cirrhosis K P Moore, G P Aithal ............................................................................................................................... Gut 2006;55(Suppl VI):vi1–vi12. doi: 10.1136/gut.2006.099580 1.0 INTRODUCTION N Grade 1 (mild). Ascites is only detectable by ultrasound examination. Ascites is a major complication of cirrhosis,1 occurring in 50% of patients over 10 years of N Grade 2 (moderate). Ascites causing moderate follow up.2 The development of ascites is an symmetrical distension of the abdomen. important landmark in the natural history of N Grade 3 (large). Ascites causing marked cirrhosis as it is associated with a 50% mortality abdominal distension. -

Procedure Coding in ICD-9-CM and ICD- 10-PCS
Procedure Coding in ICD-9-CM and ICD- 10-PCS ICD-9-CM Volume 3 Procedures are classified in volume 3 of ICD-9-CM, and this section includes both an Alphabetic Index and a Tabular List. This volume follows the same format, organization and conventions as the classification of diseases in volumes 1 and 2. ICD-10-PCS ICD-10-PCS will replace volume 3 of ICD-9-CM. Unlike ICD-10-CM for diagnoses, which is similar in structure and format as the ICD-9-CM volumes 1 and 2, ICD-10-PCS is a completely different system. ICD-10-PCS has a multiaxial seven-character alphanumeric code structure providing unique codes for procedures. The table below gives a brief side-by-side comparison of ICD-9-CM and ICD-10-PCS. ICD-9-CM Volume3 ICD-10-PCS Follows ICD structure (designed for diagnosis Designed and developed to meet healthcare coding) needs for a procedure code system Codes available as a fixed or finite set in list form Codes constructed from flexible code components (values) using tables Codes are numeric Codes are alphanumeric Codes are 3-4 digits long All codes are seven characters long ICD-9-CM and ICD-10-PCS are used to code only hospital inpatient procedures. Hospital outpatient departments, other ambulatory facilities, and physician practices are required to use CPT and HCPCS to report procedures. ICD-9-CM Conventions in Volume 3 Code Also In volume 3, the phrase “code also” is a reminder to code additional procedures only when they have actually been performed. -

AASLD Position Paper : Liver Biopsy
AASLD POSITION PAPER Liver Biopsy Don C. Rockey,1 Stephen H. Caldwell,2 Zachary D. Goodman,3 Rendon C. Nelson,4 and Alastair D. Smith5 This position paper has been approved by the AASLD and College of Cardiology and the American Heart Associa- represents the position of the association. tion Practice Guidelines3).4 Introduction Preamble Histological assessment of the liver, and thus, liver bi- These recommendations provide a data-supported ap- opsy, is a cornerstone in the evaluation and management proach. They are based on the following: (1) formal re- of patients with liver disease and has long been considered view and analysis of the recently published world to be an integral component of the clinician’s diagnostic literature on the topic; (2) American College of Physi- armamentarium. Although sensitive and relatively accu- cians Manual for Assessing Health Practices and De- rate blood tests used to detect and diagnose liver disease signing Practice Guidelines1; (3) guideline policies, have now become widely available, it is likely that liver including the AASLD Policy on the Development and biopsy will remain a valuable diagnostic tool. Although Use of Practice Guidelines and the American Gastro- histological evaluation of the liver has become important enterological Association Policy Statement on Guide- in assessing prognosis and in tailoring treatment, nonin- lines2; and (4) the experience of the authors in the vasive techniques (i.e., imaging, blood tests) may replace specified topic. use of liver histology in this setting, particularly with re- Intended for use by physicians, these recommenda- gard to assessment of the severity of liver fibrosis.5,6 Sev- tions suggest preferred approaches to the diagnostic, ther- eral techniques may be used to obtain liver tissue; a table apeutic, and preventive aspects of care. -

Chyle Leak Post Laparoscopic Cholecystectomy: a Case Report, Literature Review and Management Options
7 Case Report Page 1 of 7 Chyle leak post laparoscopic cholecystectomy: a case report, literature review and management options Ferdinand Ong1, Amitabha Das1, Kheman Rajkomar2 1Department of Upper Gastrointestinal Surgery, Liverpool Hospital, Sydney, NSW, Australia; 2Department of Upper Gastrointestinal Surgery, Bankstown-Lidcombe Hospital, Sydney, NSW, Australia Correspondence to: Dr. Kheman Rajkomar. Eldridge Road, Bankstown-Lidcombe Hospital, Bankstown NSW 2200, Sydney, Australia. Email: [email protected]. Abstract: Chyle leak after a laparoscopic cholecystectomy (LC) is very rarely reported. However, it is needs to be recognised promptly and managed as otherwise it can lead to further metabolic and infective complications. We present the case of a 48 years old man who was admitted with ultrasound proven acute calculous cholecystitis. His vital signs were within normal range but his murphy’s sign was positive. His white cell count (WCC) and liver function tests were within normal limit. He underwent an uneventful standard LC with cholangiography during the same admission with no anomalous biliary or hepatic arterial anatomy noted during the procedure. Post operatively he was noted to have 125 mL of white fluid in his drain. The fluid triglyceride was 23.2 mmol/L, cholesterol level was 2.8 mmol/L, and drain/serum triglyceride of 15.5, hence confirming it to be chyle. He was clinically otherwise very well. He was managed conservatively as a low volume chyle leak with a fat free diet. The triglyceride content in the drain effluent decreased to 1.3mmol/L by day 6 and the fluid turned straw coloured in that interval. The drain was removed and the patient discharged home without any further issues. -

Perforated Duodenal Ulcer an Alternative Therapeutic Plan
SPECIAL ARTICLE Perforated Duodenal Ulcer An Alternative Therapeutic Plan Arthur J. Donovan, MD; Thomas V. Berne, MD; John A. Donovan, MD n alternative plan for the treatment of a perforated duodenal ulcer is proposed. We will focus on the now-recognized role of Helicobacter pylori in the genesis of the ma- jority of duodenal ulcers and on the high rate of success of therapy with a combina- tion of antibiotics and a proton-pump inhibitor or histamine2 blocker in treatment of suchA ulcers. Knowledge that half the cases of perforated duodenal ulcer may have securely sealed spontaneously at the time of presentation is incorporated in the therapeutic plan. Patients with a perforated duodenal ulcer who have already been evaluated for H pylori and are not infected or, if infected, have received appropriate therapy should undergo an ulcer-definitive operation if they are suitable surgical candidates. Most authorities recommend surgical closure of the perforation and a parietal cell vagotomy. The remaining patients should have a gastroduodenogram with water- soluble contrast medium. If the perforation is sealed, the patient can be treated nonsurgically. If the perforation is leaking, secure surgical closure of the perforation is necessary. Following recov- ery from the immediate consequences of the perforation, evaluation for H pylori should be con- ducted. If the patient is infected, combined medical therapy is recommended. If the patient is not infected, Zollinger-Ellison syndrome should be ruled out and medical therapy is recommended if the ulcer has not been treated previously. Elective ulcer-definitive surgery should be considered for the occasional uninfected patient who has already received appropriate medical therapy for the ulcer. -

Postoperative Management After Hepatic Resection Lindsay J
Journal of Gastrointestinal Oncology, Vol 3, No 1, March 2012 41 Review Article Postoperative management after hepatic resection Lindsay J. Wrighton, Karen R. O’Bosky, Jukes P. Namm, Maheswari Senthil Department of Surgery, Loma Linda University, Loma Linda, California, USA ABSTRACT Hepatic resection has become the mainstay of treatment for both primary and certain secondary malignancies. Outcomes after hepatic resection have significantly improved with advances in surgical and anesthetic techniques and perioperative care. Metabolic and functional changes after hepatic resection are unique and cause significant challenges in manage- ment. In-depth understanding of hepatic physiology is essential to properly address the postoperative issues. Strategies implemented in the postoperative period to improve outcomes include adequate nutritional support, proper glycemic control, and interventions to reduce postoperative infectious complications among several others. This review article fo- cuses on the major postoperative issues after hepatic resection and presents the current management. KEY WORDS Management; postoperative; hepatic resection; liver resection J Gastrointest Oncol 2012;3:41-47. DOI: 10.3978/j.issn.2078-6891.2012.003 Introduction are further accentuated by derangements of liver function. Maintenance of adequate fluid balance and normal renal Outcomes after hepatic resection have significantly function is critical. Cirrhotics are prone to fluid shifts, improved over the last few decades (1-4). Although, no vasodilation and resultant hypotension. In this setting, single factor is solely responsible, overall advances in colloids rather than crystalloids should be administered surgical and anesthetic techniques, better understanding to restore intravascular volume. New onset postoperative of hepatic physiology and improvement in perioperative ascites frequently occurs in cirrhotic patients. -

Dysphagia to Liver Failure Vincent Ting Fung Cheung,1 Jey Singanayagam,2 Angus Molyneux,3 Neil Rajoriya1
Images in… BMJ Case Reports: first published as 10.1136/bcr-2015-212522 on 26 November 2015. Downloaded from Dysphagia to liver failure Vincent Ting Fung Cheung,1 Jey Singanayagam,2 Angus Molyneux,3 Neil Rajoriya1 1Department of DESCRIPTION Gastroenterology, Milton A 54-year-old man presenting with dysphagia, Keynes University Hospital, Milton Keynes, UK weight loss, epigastric pain and cervical lymphaden- 2Department of Radiology, opathy was referred directly to endoscopy. The Milton Keynes University medical history included hypertension and hyper- Hospital, Milton Keynes, UK 3 cholesterolaemia. Blood tests showed normal biliru- Department of bin, aspartate transaminase (AST) 490 iu/L, Histopathology, Milton Keynes γ University Hospital, Milton -glutamyl transferase (GGT) 535 iu/L, alkaline Keynes, UK phosphatase (ALP) 829 iu/L and prothrombin time (PT) 13 s. Correspondence to Gastroscopy showed a lower oesophageal lesion Dr Vincent Ting Fung Cheung, fi [email protected] ( gure 1) with biopsies showing high-grade dyspla- sia. A CT scan showed lower oesophageal wall Accepted 19 September 2015 thickening and multiple lymphadenopathy in the chest/abdomen but no liver metastases (figure 2). The local multidisciplinary team meeting outcome was for repeat oesophageal biopsies and supraclavi- cular lymph node biopsy to exclude a lymphoma. The patient thereafter clinically deteriorated over Figure 2 CT scan showing oesophageal cancer (red a 4-week period, developing jaundice. On admis- arrow) but no obvious liver lesions. sion, he became overtly encephalopathic within 48 h, with a suggestion of acute liver failure. Bloods revealed: bilirubin 238 μmol/L, AST 1034 iu/L, GGT 569 iu/L, ALP 887 iu/L, albumin 20 g/L, PT 27.2 s and lactate 4.7 mmol/L.