Recurrent, Localized Urticaria and Erythema Multiforme: a Review and Management of Cutaneous Anthrax Vaccine–Related Events
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skin Manifestation of SARS-Cov-2: the Italian Experience
Journal of Clinical Medicine Article Skin Manifestation of SARS-CoV-2: The Italian Experience Gerardo Cazzato 1 , Caterina Foti 2, Anna Colagrande 1, Antonietta Cimmino 1, Sara Scarcella 1, Gerolamo Cicco 1, Sara Sablone 3, Francesca Arezzo 4, Paolo Romita 2, Teresa Lettini 1 , Leonardo Resta 1 and Giuseppe Ingravallo 1,* 1 Section of Pathology, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] (G.C.); [email protected] (A.C.); [email protected] (A.C.); [email protected] (S.S.); [email protected] (G.C.); [email protected] (T.L.); [email protected] (L.R.) 2 Section of Dermatology and Venereology, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] (C.F.); [email protected] (P.R.) 3 Section of Forensic Medicine, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] 4 Section of Gynecologic and Obstetrics Clinic, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] * Correspondence: [email protected] Abstract: At the end of December 2019, a new coronavirus denominated Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified in Wuhan, Hubei province, China. Less than three months later, the World Health Organization (WHO) declared coronavirus disease-19 (COVID-19) to be a global pandemic. Growing numbers of clinical, histopathological, and molecular findings were subsequently reported, among which a particular interest in skin manifestations during the course of the disease was evinced. Today, about one year after the development of the first major infectious foci in Italy, various large case series of patients with COVID-19-related skin Citation: Cazzato, G.; Foti, C.; manifestations have focused on skin specimens. -

Erythema Marginatum
Figurative Erythemas Michelle Goedken, DO Affiliated Dermatology Scottsdale, AZ Figurative Erythemas • Erythema annulare centrifugum • Erythema marginatum • Erythema migrans • Erythema gyratum repens • Erythema multiforme Erythemas • Erythemas represent a change in the color of the skin that is due to the dilation of blood vessels, especially those in the papillary and reticular dermis • The color is blanchable and most last for days to months • Figurative erythemas have an annular, arciform or polycyclic appearance ERYTHEMA ANNULARE CENTRIFUGUM ERYTHEMA ANNULARE CENTRIFUGUM • Pathogenesis: EAC represents a reaction pattern or hypersensitivity to one of many antigens – IL-2 and TNF-alpha may have a role – Most patients do not have an underlying disease identified ERYTHEMA ANNULARE CENTRIFUGUM • Associated with: – Infection » Dermatophytes and other fungi (Candida and Penicillium in blue cheese) » Viruses: poxvirus, EBV, VZV, HIV » Parasites and ectoparasites – Drugs: diuretics, antimalarials, gold, NSAIDs, finasteride, amitriptyline, etizolam, Ustekinumab (2012) ERYTHEMA ANNULARE CENTRIFUGUM – Foods – Autoimmune endocrinopathies – Neoplasms (lymphomas and leukemias) – Pregnancy – Hypereosinophilic syndrome – Lupus (2014) ERYTHEMA ANNULARE CENTRIFUGUM http://www.dermaamin.com Rongioletti, F., Fausti, V., & Parodi, A ERYTHEMA ANNULARE CENTRIFUGUM • 2 major forms: – Superficial: classic trailing scale, may have associated pruritus – Deep: infiltrated borders, usually no scale, edges are elevated, usually not pruritic ERYTHEMA ANNULARE CENTRIFUGUM -

Diagnosis, Classification, and Management of Erythema
Arch Dis Child 2000;83:347–352 347 Diagnosis, classification, and management of Arch Dis Child: first published as 10.1136/adc.83.4.347 on 1 October 2000. Downloaded from erythema multiforme and Stevens–Johnson syndrome C Léauté-Labrèze, T Lamireau, D Chawki, J Maleville, A Taïeb Abstract become widely accepted that EM and SJS, as Background—In adults, erythema multi- well as toxic epidermal necrolysis, are all part of forme (EM) is thought to be mainly a single “EM spectrum”. In both EM and SJS, related to herpes infection and Stevens– pathological changes in the earliest skin lesion Johnson syndrome (SJS) to drug reac- consist of the accumulation of mononuclear tions. cells around the superficial dermal blood Aims—To investigate this hypothesis in vessels; epidermal damage is more characteris- children, and to review our experience in tic of EM with keratinocyte necrosis leading to the management of these patients. multilocular intraepidermal blisters.5 In fact, Methods—A retrospective analysis of 77 there is little clinical resemblance between paediatric cases of EM or SJS admitted to typical EM and SJS, and recently some authors the Children’s Hospital in Bordeaux be- have proposed a reconsideration of the “spec- tween 1974 and 1998. trum” concept and a return to the original Results—Thirty five cases, inadequately description.15–17 According to these authors, the documented or misdiagnosed mostly as term EM should be restricted to acrally urticarias or non-EM drug reactions were distributed typical targets or raised oedema- excluded. Among the remaining 42 pa- tous papules. Depending on the presence or tients (14 girls and 28 boys), 22 had EM (11 absence of mucous membrane erosions the EM minor and 11 EM major), 17 had SJS, cases may be classified as EM major or EM 16 and three had isolated mucous membrane minor. -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

Isotretinoin and the Risk of Erythema Multiforme Final SPC and PL
Isotretinoin and the risk of erythema multiforme Final SPC and PL wording agreed by the PhVWP in June 2010 Doc. Ref.: CMDh/PhVWP/021/2010 Rev 1 July 2010 SUMMARY OF PRODUCT CHARACTERISTICS Section 4.4 There have been post-marketing reports of severe skin reactions (e.g. erythema multiforme (EM), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)) associated with isotretinoin use. As these events may be difficult to distinguish from other skin reactions that may occur (see section 4.8), patients should be advised of the signs and symptoms and monitored closely for severe skin reactions. If a severe skin reaction is suspected, isotretinoin treatment should be discontinued. Section 4.8 Skin and subcutaneous tissues Cheilitis, dermatitis, dry skin, localised exfoliation, pruritus, rash disorders: erythematous, skin fragility (risk of frictional trauma) Very common (≥ 1/10) Rare (≥ 1/10 000,< 1/1000) Alopecia Acne fulminans, acne aggravated (acne flare), erythema (facial), exanthema, hair disorders, hirsutism, nail dystrophy, paronychia, Very Rare (≤ 1/10 000) photosensitivity reaction, pyogenic granuloma, skin hyperpigmentation, sweating increased, ∗ Erythema multiforme, Stevens-Johnson Syndrome, toxic Unknown epidermal necrolysis. PACKAGE LEAFLET Possible side effects Skin and hair problems Unknown frequency Serious skin rashes (erythema multiforme, Stevens- Johnson syndrome, and toxic epidermal necrolysis), which are potentially life-threatening and require immediate medical attention. These appear initially as circular patches often with central blisters usually on arms and hands or legs and feet, more severe rashes may include blistering of the chest and back. Additional symptoms such as infection of the eye (conjunctivitis) or ulcers of the mouth, throat or nose may occur. -

04Amersoncommondermatoses
Conflicts of Interest None Common Dermatoses in Children & Adults Erin Amerson, MD Department of Dermatology UC San Francisco Outline Impetigo Infections & Infestations Organism 50-70% staphylococcus aureus Remainder group A beta- Skin cancer hemolytic streptococcus or both 2 Forms: Common dermatologic disorders Honey-colored crusts Less common but important diseases Bullous Impetigo- staphylococcus 1 Impetigo Treatment Systemic Abx + topical therapy is best Soak off thick crusts, may use mupirocin oint Beta-lactamase resistant antibiotics x 7 days Dicloxacillin Cephalexin To eradicate nasal Staph carriage Rifampin 600 mg qd X 5 days with your other Abx OR Mupirocin (Bactroban) to nares bid Methicillin Resistant Staph Aureus (MRSA) 40-59% MRSA at UCSF/SFGH Culture for organism and sensitivities Consider if recurrent infection Oral antibiotics that still work: Doxycycline or minocycline Trimethoprim-sulfamethoxazole Clindamycin Can combine any of the above with rifampin Save IV Vanco or Linezolid for MRSA resistant to EVERYTHING 2 Groin Fold Rash DDx Tinea cruris Seborrheic dermatitis Erythrasma Intertrigo Candida Inverse psoriasis Fungal/Yeast Infections of the Groin Tinea Cruris Dermatophyte and Scaly, crusted plaque with central clearing Nystatin not effective Yeast Infections Imidizole/Allylamines x 2-4 weeks as for tinea corporis Candida Moister, more red, satellite pustules Drying agents like Domeboro’s soaks, then Nystatin/Imidizoles 3 Treatment of Onychomycosis Trichophyton rubrum Why treat? Confirm fungal infection before treating DDx: psoriasis, trauma, lichen planus No longer use Griseofulvin: 12-18 months rx & poor efficacy Ketoconazole: risk ↑ LFT’s with long-term use Treatment of Onychomycosis Nail Psoriasis Terbinafine (Lamisil) 250 mg/day x 3-4 months Pulsing being studied Liver toxicity Itraconazole (Sporonox) Pulse at 400 mg/day x 7 days/ mo x 3 months Drug-drug interactions Liver toxicity/CHF/$$$$ 4 Tinea Capitis Tinea Capitis Treatment What to look for: p.o. -

Oral Diseases Associated with Human Herpes Viruses: Aetiology, Clinical Features, Diagnosis and Management
www.sada.co.za / SADJ Vol 71 No. 6 CLINICAL REVIEW < 253 Oral diseases associated with human herpes viruses: aetiology, clinical features, diagnosis and management SADJ July 2016, Vol 71 no 6 p253 - p259 R Ballyram1, NH Wood2, RAG Khammissa3, J Lemmer4, L Feller5 ABSTRACT Human herpesviruses (HHVs) are very prevalent DNA ACRONYMS viruses that can cause a variety of orofacial diseases. EM: erythema multiforme Typically they are highly infectious, are contracted early in HHV: human herpes virus life, and following primary infection, usually persist in a latent form. Primary oral infections are often subclinical, but may PCR: polymerase chain reaction be symptomatic as in the case of herpes simplex virus- HSV, HHV-1: herpes simplex virus induced primary herpetic gingivostomatitis. Reactivation VZV, HHV-3: varicella-zoster virus of the latent forms may result in various conditions: herpes EBV, HHV-4: Epstein-Barr virus simplex virus (HSV) can cause recurrent herpetic orolabial CMV, HHV-5: cytomegalovirus lesions; varicella zoster virus (VZV) can cause herpes zoster; Epstein-Barr virus (EBV) can cause oral hairy Key words: herpes simplex virus, human herpes virus-8, leukoplakia; and reactivation of HHV-8 can cause Kaposi varicella zoster virus, Epstein-Barr virus, recurrent herpes sarcoma. In immunocompromised subjects, infections labialis, recurrent intraoral herpetic ulcers, treatment, val- with human herpesviruses are more extensive and aciclovir, aciclovir, famcicylovir. severe than in immunocompetent subjects. HSV and VZV infections are treated with nucleoside analogues aciclovir, valaciclovir, famciclovir and penciclovir. These agents INTRODucTION have few side effects and are effective when started The human herpesvirus (HHV) family comprises a diverse early in the course of the disease. -

Erythema Multiforme: Recognition and Management Kathryn P
Erythema Multiforme: Recognition and Management Kathryn P. Trayes, MD; Gillian Love, MD; and James S. Studdiford, MD Thomas Jefferson University Hospital, Philadelphia, Pennsylvania Erythema multiforme is an immune-mediated reaction that involves the skin and sometimes the mucosa. Classically described as target-like, the erythema multiforme lesions can be isolated, recur- rent, or persistent. Most commonly, the lesions of erythema multiforme present symmetrically on the extremities (especially on extensor surfaces) and spread centripetally. Infections, especially herpes simplex virus and Mycoplasma pneumoniae, and medications constitute most of the causes of erythema multiforme; immunizations and autoimmune diseases have also been linked to erythema multiforme. Erythema multiforme can be differentiated from urticaria by the duration of individual lesions. Ery- thema multiforme lesions are typically fixed for a minimum of seven days, whereas individual urticarial lesions often resolve within one day. Erythema multiforme can be confused with the more serious con- dition, Stevens-Johnson syndrome; however, Stevens-Johnson syndrome usually contains widespread erythematous or purpuric macules with blisters. The management of erythema multiforme involves symptomatic treatment with topical steroids or antihistamines and treating the underlying etiology, if known. Recurrent erythema multiforme associated with the herpes simplex virus should be treated with prophylactic antiviral therapy. Severe mucosal erythema multiforme can require hospitalization -

Exanthems and Drug Reactions
Dermatology Exanthems and Morton Rawlin drug reactions ‘Well, Mr Jones, I think we should put you on this tablet to Background fix this problem. Now, the things you need to look out for Drug reactions are a common cause of rashes and can vary are any rashes…’ from brief, mildly annoying, self limiting rashes to severe conditions involving multiple organ systems. How often in general practice do you hear yourself Objective offering this advice? Why do almost all drugs list rash as This article outlines an approach to exanthems that a side effect? How do they occur and what can you do to may be related to drug reactions and details appropriate recognise and manage them? management. The skin is the largest organ of the body and, from a diagnostic Discussion viewpoint, we can see it change to various stimuli. Medications Rashes related to drug reactions are both nonallergic and allergic. Nonallergic rashes are usually predictable and are commonly used and are integral to the general practitioner’s may be avoidable. Allergic rashes include morbilliform armamentarium for treating most ills. However, it is also important erythema, urticaria and angioedema, erythema multiforme to note that increasing access to medications by consumers through and vasculitic rashes. The vast majority of cases are other health professionals (eg. naturopaths) and the self prescribed rapidly resolving and self limiting once the offending use of over-the-counter, complementary and alternative medicines agent is removed. Early recognition and supportive should be remembered in the history taking of a patient presenting measures are the keys to care in the majority of cases. -
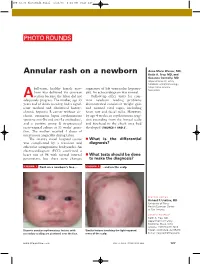
Annular Rash on a Newborn Keith A
JFP_02.06_PhotoRnds.Final 1/23/06 1:31 PM Page 127 PHOTO ROUNDS Anne-Marie Warner, MD, Annular rash on a newborn Keith A. Frey, MD, and Suzanne Connolly, MD Departments of Family Medicine and Dermatology, Mayo Clinic Arizona, full-term, healthy female new- suggestive of left ventricular hypertro- Scottsdale born was delivered via cesarean phy. An echocardiogram was normal. A section because the labor did not Follow-up office visits for com- adequately progress. The mother, age 33 mon newborn feeding problems years and of Asian ancestry, had a signif- demonstrated consistent weight gain icant medical and obstetrical history: and normal vital signs, including chronic hepatitis B carrier without cir- heart rate and facial milia. However, rhosis, cutaneous lupus erythematosus by age 4 weeks an erythematous erup- (positive anti-Ro and anti-La antibodies), tion extending from the frontal scalp and a positive group B streptococcal and forehead to the cheek area had recto-vaginal culture at 35 weeks’ gesta- developed (FIGURES 1 AND 2). tion. The mother received 4 doses of intravenous ampicillin during labor. The infant’s initial hospital course ■ What is the differential was complicated by a transient and diagnosis? otherwise asymptomatic bradycardia. An electrocardiogram (ECG) confirmed a heart rate of 96 with normal interval ■ What tests should be done parameters, but there were changes to make the diagnosis? FIGURE 1 Rash on a newborn’s face ... FIGURE 2 ...and on the scalp FEATURE EDITOR Richard P. Usatine, MD University of Texas Health Sciences Center at San Antonio CORRESPONDENCE Keith A. Frey, MD, Department of Family Medicine, Mayo Clinic Arizona, 13737 North 92nd Discrete annular erythematous lesions with Similar lesions involving the light-exposed Street, Scottsdale, AZ 85260. -

“Drug Induced Erythema Multiforme” – a Review Dr
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 10, 2020 “Drug Induced Erythema Multiforme” – A Review Dr. Sudakshina Mukherjee, Dr. N. Aravindha Babu., Dr. L.Malathy, Dr. N.Anitha Post graduate student. Department of Oral pathology and Microbiology Sree Balaji Dental College and Hospital and Research Bharath Institute of Higher Education and Research ABSTRACT: Erythema Multiforme (EM) is an uncommon, acute inflammatory reactive mucocutaneous disorder. It's a hypersensitivity reaction to different antigenic stimuli, most common being infection followed by drugs. It occurs predominantly in younger age group. Recurrence in EM can be seen especially secondary to HSV infection. Erythema multiforme is manifested clinically as classical cutaneous target like lesions and mucosal bullae or erosions. Within the total affected patients 70% of them suffer from mucosal involvement though mucosal involvement separately is comparatively rare. Manifestations of EM can be varying and may pose a diagnostic dilemma because infections (particularly herpes simplex and mycoplasma pneumoniae) and drugs seem to predispose towards development of EM. KEYWORDS: erythema multiforme, Stevens and Johnson, target lesion, bilateral conjunctivitis INTRODUCTION: Bateman and Bulkley in the year of 1817 first recognized EM, But in the year 1846, the first American case “Herpes Iris.” was reported. Later, in 1866 under the term “erythema exsudativum multiforme” first characteristic morphological feature of this particular eruptions was described by Hebra[1] along with its cause which was due to internal or systemic origin and not for any local etiology [2]. Stevens and Johnson, [3] in 1922, reported EM with predominant involvement of the oral and conjunctival mucous membranes as “a new eruptive fever associated with stomatitis and ophthalmia.” On systemic drug administration severe reactions are manifested which characterizes like EM, and Steven Johnson syndrome. -

Urticaria Multiforme: a Benign Frightening Rash Mariana Barros , Joana Antunes, Sofia Moura Antunes, Rita Calado
Images in… BMJ Case Rep: first published as 10.1136/bcr-2020-241011 on 28 January 2021. Downloaded from Urticaria multiforme: a benign frightening rash Mariana Barros , Joana Antunes, Sofia Moura Antunes, Rita Calado Department of Pediatrics, DESCRIPTION Skin lesions start with papules that expand to Hospital de Cascais, Dr José de We report the case of a previously healthy form annular, polycyclic, erythematous wheals Almeida, Cascais, Alcabideche, 13- month- old boy who developed transient, annular with dusky, ecchymotic centres affecting the Portugal and erythematous wheals with dusky centres trunk, extremities and face.1 3 Face and extrem- affecting the face, trunk and limbs, associated with ities oedema, pruritus and dermographism are Correspondence to also present in most patients. Children present an Dr Mariana Barros; pruritus, non- pitting oedema of upper and lower marianaantunesbarros@ gmail. extremities and dermographism (figure 1). The rash non- toxic appearing and systemic symptoms are com spared palms and soles and disappeared with digital commonly limited to a few days of mild fever.3–6 In pressure and it had started 7 days after immunisation addition to presenting all the characteristic features Accepted 13 January 2021 for measles–mumps–rubella, meningococcal type C of this disease on examination, our patient met the and pneumococcal conjugate vaccine. The child was age range for this condition as well as a known haemodynamically stable, had good overall condi- common trigger like recent immunisations. tion and no other symptoms besides a low fever and Urticaria multiforme is commonly misdiagnosed an otherwise normal physical examination without for erythema multiforme, serum sickness- like reac- the presence of petequiae or purpura, acute respi- tion, urticarial vasculitis, among others, despite ratory distress, laryngoedema, angioedema, lymph- being more frequent than the previous conditions.1 adenopathy, hepatosplenomegaly, arthralgia or However, true target lesions, skin necrosis, blis- mucous membranes involvement.