Drug Solid Solutions–A Method for Tuning Phase Transformations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Winter for the Membership of the American Crystallographic Association, P.O
AMERICAN CRYSTALLOGRAPHIC ASSOCIATION NEWSLETTER Number 4 Winter 2004 ACA 2005 Transactions Symposium New Horizons in Structure Based Drug Discovery Table of Contents / President's Column Winter 2004 Table of Contents President's Column Presidentʼs Column ........................................................... 1-2 The fall ACA Council Guest Editoral: .................................................................2-3 meeting took place in early 2004 ACA Election Results ................................................ 4 November. At this time, News from Canada / Position Available .............................. 6 Council made a few deci- sions, based upon input ACA Committee Report / Web Watch ................................ 8 from the membership. First ACA 2004 Chicago .............................................9-29, 38-40 and foremost, many will Workshop Reports ...................................................... 9-12 be pleased to know that a Travel Award Winners / Commercial Exhibitors ...... 14-23 satisfactory venue for the McPherson Fankuchen Address ................................38-40 2006 summer meeting was News of Crystallographers ...........................................30-37 found. The meeting will be Awards: Janssen/Aminoff/Perutz ..............................30-33 held at the Sheraton Waikiki Obituaries: Blow/Alexander/McMurdie .................... 33-37 Hotel in Honolulu, July 22-27, 2005. Council is ACA Summer Schools / 2005 Etter Award ..................42-44 particularly appreciative of Database Update: -

Publishing Catalogue 2016 Royal Society of Chemistry RSC Gold Everything You Need in One Sparkling Online Package
Publishing Catalogue 2016 Royal Society of Chemistry RSC Gold Everything you need in one sparkling online package RSC Gold is: • Balanced and complete – stay connected to all the key content within the chemical sciences spectrum • International – authors and editorial boards from over one hundred countries contribute, ensuring a truly global perspective • Flexible – we offer tiered pricing options based on your institution’s size and scope, no matter how big or small RSC Gold includes: • A wide-ranging list of Royal Society of Chemistry Caltech’s RSC Gold journal, database and magazine content package subscription has • Our Journals Archive lease 1841-2007 been a very welcome development ... I am very • A bonus eBook series appreciative of the general excellence of articles in the • The option to continually upgrade and expand your RSC research journals, subscription with new journals and our latest published evidenced by strong impact factors and material increases in local download statistics. There’s also Gold for Gold… Dana L Roth Caltech Library, US Purdue University, USA Gold for Gold voucher codes can be used to publish new chemical science papers in any Royal Society of Chemistry journal via the Gold open access option. Each voucher code can be used to publish one paper free of charge, and is a good way to ensure maximum visibility for your institution’s research. Find out more – contact your Account Manager or email [email protected] Registered charity number: 207890 Royal Society of Chemistry | Publishing | www.rsc.org/publishing Contacts For contact details please see the Regional Representatives and Agents list on page 40. -

Chemistry Subject Ejournal Packages
Chemistry subject eJournal packages Subject Included journals No. of journals Analyst; Biomaterials Science; Food & Function; Journal of Materials Chemistry B; Lab on a Chip; Metallomics; Molecular Omics; Biological chemistry Molecular Systems Design & Engineering; Photochemical & Photobiological Sciences; Toxicology Research 10 Catalysis Science & Technology; Dalton Transactions; Energy & Environmental Science; Green Chemistry; Organic & Biomolecular Chemistry; Catalysis science Photochemical & Photobiological Sciences; Physical Chemistry Chemical Physics; Reaction Chemistry & Engineering 8 Lab on a Chip; MedChemComm; Metallomics; Molecular Omics; Natural Product Reports; Organic & Biomolecular Chemistry; Photochemical Biochemistry & Photobiological Sciences; Toxicology Research 8 CrystEngComm; Energy & Environmental Science; Green Chemistry; Journal of Materials Chemistry A; Molecular Systems Design & Energy Engineering; Physical Chemistry Chemical Physics; Journal of Materials Chemistry 7 Energy & Environmental Science; Environmental Science: Nano; Environmental Science: Processes & Impacts; Environmental Science: Environmental Science Water Research & Technology; Green Chemistry; Journal of Materials Chemistry A & C; Photochemical & Photobiological Sciences; Reaction 8 Chemistry & Engineering Food science Analyst; Analytical Methods; Food & Function; Lap on a Chip 4 Catalysis Science & Technology; CrystEngComm; Dalton Transactions; Inorganic Chemistry Frontiers; Metallomics; Photochemical & Inorganic chemistry Photobiological Sciences; -

RSC Gold 2015 Flyer.Pdf
RSC Gold Want access to full content from the world’s leading chemistry society? Including regular new material and an Archive dating back to 1841? Caltech’s RSC Gold Plus voucher codes to publish package subscription has been a very Open Access (OA) free of charge? welcome development ... I am very appreciative of the RSC Gold is the Royal Society of Chemistry’s general excellence of articles in the RSC premium package comprising 41 international research journals, evidenced by strong journals, literature updating services and impact factors and magazines that will meet the needs of all your increases in local download statistics. end-users. And the accompanying Gold for Gold Dana L. Roth OA voucher codes ensure maximum visibility for Chemistry Librarian your institution’s quality research. Caltech, USA Take a look inside to see exactly what you get www.rsc.org/gold RSC Gold includes a wealth of quality RSC journal, database and magazine content that is all available online. Journals Natural Product Reports Analyst New Journal of Chemistry Analytical Methods Organic & Biomolecular Chemistry Biomaterials Science Photochemical & Photobiological Sciences Catalysis Science & Technology Physical Chemistry Chemical Physics (PCCP) Chemical Communications Polymer Chemistry Chemical Science* RSC Advances Chemical Society Reviews Soft Matter CrystEngComm Toxicology Research Dalton Transactions Energy & Environmental Science B a c k fi l e Environmental Science: Nano** RSC Journals Archive 1841-2007 lease Environmental Science: Processes & Impacts -

Publishing Update 2020
Publishing update 2020 We are an international, not-for-profit organisation connecting chemical scientists with each other, with other scientists, and with society as a whole. Founded in 1841 and based in London, UK, we have a high profile reputation as an international publisher with 46 peer-reviewed journals, more than 1,500 print books and a collection of online databases and literature updating services. We are here to give every mind in the chemical sciences the information and tools to try something new – to help everyone feel confident enough to challenge the accepted and get a step closer to answering fundamental questions. Registered charity number: 207890 New from our journals Journal family updates Horizons The Horizons journals publish short reports of They are focal points for research that pushes exceptional high quality and innovation. the boundaries and challenges current thinking. Volume 5 Volume 2 Number 3 Number 1 May 2018 January 2017 Materials Pages 311-580 Nanoscale Pages 1-66 Horizons Horizons The home for rapid reports of exceptional significance in nanoscience and nanotechnology rsc.li/materials-horizons rsc.li/nanoscale-horizons IMPACT IMPACT FACTOR FACTOR ISSN 2051-6347 ISSN 2055-6756 COMMUNICATION PAPER Blaise L. Tardy, Orlando J. Rojas et al. Xiao Cheng Zeng, Hui Zhao et al. Biofabrication of multifunctional nanocellulosic 3D structures: Type-I van der Waals heterostructure formed by MoS2 and a facile and customizable route 14.356* ReS2 monolayers 9.095* Materials Horizons Nanoscale Horizons Materials Horizons publishes exceptionally high quality, Nanoscale Horizons is the home for exceptionally high quality, innovative materials science, placing emphasis on original innovative nanoscience & nanotechnology. -

Publishing Price List 2016
Publishing Price List 2016 Royal Society of Chemistry Collections for 2016 RSC GOLD INCLUDES: Key Royal Society of Chemistry online Price on application ONLINE ONLY† journal, database and magazine content, plus a EMAIL [email protected] book series. or contact your Account Manager PRICES JOURNALS ARCHIVE ONLINE ONLY† RSC Journals Archive Outright Purchase (1841 – 2007) • £41,097 • $72,615 RSC Journals Archive Outright Purchase (2005 – 2007) • £4,867 • $8,271 RSC Journals Archive Lease (1841 – 2007) • £7,408 • $13,133 PRICES RSC Journals Archive Hosting Fee • £819 • $1,340 THE HISTORICAL COLLECTION INCLUDES: Price on application ONLINE ONLY† • Society Publications (1949 – 2012) EMAIL [email protected] • Society Minutes (1841 – 1966) or contact your Account Manager • Historical Papers (1505 – 1991) PRICES CORE CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Dalton Transactions • Journal of Materials Chemistry A, B & C • New Journal of Chemistry PRINT & ONLINE† ONLINE ONLY† • Organic & Biomolecular Chemistry • • • Physical Chemistry Chemical Physics £19,685 £18,701 • RSC Advances (online only) • $36,814 • $34,973 PRICES GENERAL CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Chemical Society Reviews PRINT & ONLINE† ONLINE ONLY† • Chemistry World • £8,360 • £7,942 • New Journal of Chemistry PRICES • $13,685 • $13,244 • RSC Advances (online only) ANALYTICAL SCIENCE COLLECTION INCLUDES: • Analyst • Analytical Abstracts (online only) • Analytical Methods PRINT & ONLINE† ONLINE ONLY† • Environmental Science: Processes & Impacts • -

Pliego De Contratación 90/2015
PLIEGO DE CONTRATACIÓN 90/2015 Documento Página/s ÍNDICE 1 PLIEGO DE CLÁUSULAS 2-24 ADMINISTRATIVAS PARTICULARES PLIEGO PRESCRIPCIONES TÉCNICAS 25-26 ANEXO LISTADO PAQUETES 27-124 El presente pliego ha sido informado favorablemente por la asesoría Jurídica de la Universidad de Murcia con fecha 05/11/2015 Firmante: VICERRECTOR DE INFRAESTRUCTURAS Y SOSTENIBILIDAD UNIVERSIDAD MURCIA - MURCIA; Fecha-hora: 17/11/2015 12:49:39; Emisor del certificado: C=ES,O=ACCV,OU=PKIACCV,CN=ACCVCA-120; Código seguro de verificación: RVhQMNgW-bsiQbt8i-2xOHXV87-UM/Ptelz Página 1 de 124 Este es un documento administrativo electrónico emitido por la Universidad de Murcia. Su autenticidad puede ser contrastada a través de la siguiente dirección: https://sede.um.es/validador/ Documento: PLIEGO DE CLÁUSULAS ADMINISTRATIVAS PARTICULARES Órgano de Contratación El Rector, P.D. (Resolución R-392/2014 de 21 mayo), El Gerente. Nº de expediente: 2015/90/PR-AM Tipo de contrato: Privado. Objeto: Suministro de recursos digitales de información científica disponibles en paquetes editoriales Lotes: Sí (9 lotes) Tramitación del expediente: Ordinaria (artículos 109 y 110 TRLCSP). Tramitación anticipada Procedimiento adjudicación: Abierto (artículos 138 y 157 del TRLCSP) Regulación armonizada: Sí Presupuesto de licitación: 563.400,35 euros (IVA -21%-: 118.314,05 €; Total: 681.714,40 €) Valor estimado: 563.400,35 euros PLIEGO DE CLÁUSULAS ADMINISTRATIVAS PARTICULARES PARA LA CONTRATACIÓN DE SUMINISTROS. I. ELEMENTOS DEL CONTRATO 1. OBJETO, FINALIDAD Y NECESIDAD DEL CONTRATO 1.1. El objeto y destino de este contrato de suministro se describen en el apartado A) del Cuadro Resumen que se incluye al final de este pliego. -

RSC Gold 2017
RSC Gold 2017 RSC Gold is the Royal Society of Chemistry’s premium online package made up of 36 chemical science journals, an eBook series and five literature updating services. An RSC Gold subscription ensures your library has... ...access to high quality chemistry research Our journal portfolio has an average Impact factor of 6.105* and RSC Gold gives access to leading chemistry journals including: Volume 45 Number 1 7 January 2016 Pages 1–226 Volume 9 Number 1 January 2016 Pages 1–268 Volume 3 Number 1 January 2016 Pages 1–84 Volume 33 Number 1 January 2016 Pages 1–112 Energy & | Environmental Materials Natural Product Chem Soc Rev Science | 20 6 Horizons Reports Chemical Society Reviews www.rsc.org/chemsocrev www.rsc.org/ees rsc.li/materials-horizons www.rsc.org/npr M il H i ISSN 0306-0012 IMPACT ISSN 1754-5692 ISSN 2051-6347 IMPACT ISSN 0265-0568 IMPACT PAPER #1 REVIEW ARTICLE Félix Urbain et al. COMMUNICATION REVIEW ARTICLE Timothy Noël et al. FACTOR Multijunction Si photocathodes with tunable photovoltages from 2.0 V to 8 Stefan A. F. Bon et al. FACTOR Ronald J. Quinn et al. FACTOR Liquid phase oxidation chemistry in continuous-fl ow microreactors 2.8 V for light inducedENVIRONM water splitting ENTAL Control of vesicle membrane permeability with catalytic particles TCM, brain function and drug space SCIENCes 34.09* JOURNAL 9.095 * 10.986* *Data based on 2015 Journal Citation Reports® (Thomson Reuters, June 2016) ...a comprehensive collection of resources and chemical sciences research Journals ( ) included in RSC Gold cover the breadth of chemistry and related fields.** Analytical General chemistry Biological Inorganic Catalysis Materials Chemical biology & medicinal Nanoscience Energy Organic Environmental Physical Food **please note some journals are counted in multiple subject areas ...a truly global perspective Authors and editorial boards from more than 100 countries contribute to our content. -

The Royal Society of Chemistry Digital Library Conference , Slovakia 2015 Claudia Heidrich Inside Sales Executive Central, Eastern & Northern Europe Content
The Royal Society of Chemistry Digital Library Conference , Slovakia 2015 Claudia Heidrich Inside Sales Executive Central, Eastern & Northern Europe Content • Royal Society of Chemistry ( Publishing Portfolio and Scope ) • RSC’s Development • Input from Slovak and Czech Authors • Open Access @ RSC and results in 2014 Royal Society of Chemistry (RSC) • Founded in 1841 • One of the largest organisations for advancing the chemical sciences • Not-for-profit Organisation with a worldwide network of over 51,000 members The Royal Society of Chemistry Advancing excellence in the chemical sciences Learned Society International Charity Education not-for profit Facilitator Publisher Science Policy Conferences & RSC - campaigning Activities Events organisation Professional Global Membership Body Organisation Qualifications Library and Information Centre Royal Society of Chemistry London & Cambridge Washington D.C. Berlin & Philadelphia Tokyo Beijing & Bangalore Shanghai São Paulo A global community What do we publish? • Books and eBooks • Journals • Databases • Literature Updating Services • Magazines • Major Reference Works What is our scope? • Chemical Sciences • Energy & Environmental Sciences • Food Science • Medicinal Chemistry & Biomolecular Sciences • Nano, Polymers & Materials Science Our growth In 2014, we published 36,000 articles, that’s almost 6 times as many articles as we delivered in 2008. Our share of the market has tripled since 2008. Our international influence In 2014, over 113,000 authors from 85 countries published with us, and -
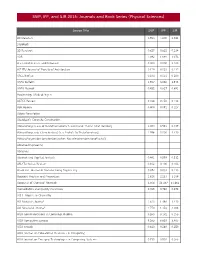
SNIP, IPP, and SJR 2015: Journals and Book Series (Physical Sciences)
SNIP, IPP, and SJR 2015: Journals and Book Series (Physical Sciences) Source Title SNIP IPP SJR 2D Materials 0.925 5.200 4.344 3 Biotech 3D Research 0.627 0.632 0.214 4OR 1.092 1.094 1.073 A + U-Architecture and Urbanism 0.000 0.000 0.100 A|Z ITU Journal of Faculty of Architecture 0.124 0.053 0.112 AACL Bioflux 0.474 0.351 0.200 AAPG Bulletin 1.907 3.330 1.978 AAPG Memoir 0.452 0.627 0.691 Aardkundige Mededelingen AATCC Review 0.266 0.250 0.134 ABB Review 0.404 0.091 0.127 Abbey Newsletter Aberdeen's Concrete Construction Abhandlungen aus dem Mathematischen Seminar der Universitat Hamburg 1.054 0.541 0.447 Abhandlungen der Senckenberg Gesellschaft fur Naturforschung 1.996 5.000 1.430 Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft Abrasive Engineering Abrasives Abstract and Applied Analysis 0.441 0.579 0.512 ABU Technical Review 0.102 0.100 0.103 Academic Journal of Manufacturing Engineering 0.072 0.033 0.113 Accident Analysis and Prevention 1.628 2.213 1.109 Accounts of Chemical Research 4.506 21.467 11.465 Accreditation and Quality Assurance 0.965 0.740 0.378 ACH - Models in Chemistry ACI Materials Journal 1.620 1.446 1.430 ACI Structural Journal 1.750 1.354 2.088 ACM Communications in Computer Algebra 0.263 0.172 0.153 ACM Computing Surveys 8.265 6.633 3.405 ACM Inroads 0.630 0.248 0.250 ACM Journal on Educational Resources in Computing ACM Journal on Emerging Technologies in Computing Systems 0.735 0.932 0.366 Source Title SNIP IPP SJR ACM SIGPLAN Notices 0.803 0.571 0.490 ACM Transactions on Accessible Computing -

Royal Society of Chemistry(RSC/英国王立科学会)
Royal Society of Chemistry(RSC/英国王立科学会) 2017/4月 Notes Title Year 1 Analyst 1997-present 2 Analytical Abstracts 1980-present 3 Analytical Communications 1997-1999 4 Analytical Methods 2009-present 5 Annual Reports on the Progress of Chemistry, Sect. A 1997-2013 6 Annual Reports on the Progress of Chemistry, Sect. B 1997-2013 7 Annual Reports on the Progress of Chemistry, Sect. C 1997-2013 8 Biomaterials Science 2013-present 9 Catalysis Science & Technology 2010-present 10 Chemical Communications (Cambridge) 1997-present 11 Free Chemical Science 2010-present 12 Chemical Society Reviews 1997-present 13 Chemistry Education Research and Practice 2008-present 14 Contemporary Organic Synthesis 1997-1997 15 CrystEngComm 1999-present 16 Dalton Transactions 2003-present 17 Education in chemistry 2004-present 18 Energy & Environmental Science 2008-present 19 Environmental Science: Nano 2014-present 20 Environmental Science: Processes & Impacts 2013-present 21 Environmental Science: Water Research & Technology 2015-present 22 Faraday Discussions 1997-present 23 Food & Function 2010-present 24 Geochemical Transactions 2000-2003 25 Green Chemistry 1999-present 26 Integrative Biology 2009-present 27 e-book Issues in Environmental Science and Technology 1994-present 28 Journal of Analytical Atomic Spectrometry 1997-present 29 Journal of Chemical Research, Synopses 1997-1999 30 Journal of Environmental Monitoring 1999-2012 31 Journal of Materials Chemistry 1997-2012 32 Journal of Materials Chemistry A: Materials for energy and 2013-present sustainability -

Electronic Access Licence Agreement
ELECTRONIC ACCESS LICENCE AGREEMENT This Agreement is made the Chemistry located at ("Publisher"} and Th offices at WHEREAS (A) Publisher holds journal articles and databases in electronic form; (B) Customer wishes to license access to journal articles and/or databases; and (C) Publisher agrees to grant such licence. NOW, THEREFORE, in consideration of the mutual promises set forth herein, the parties agree as follows: 1. Definitions In thisAgreement the following terms shall have the following meanings: "Authorised Users" means current employees (including faculty, staff, and independent contractors) of the Customer and students ofthe Customer who, in either case, have been allowed access to Publlsher Content by Customer, regardless of the physical location of such persons, such access shall be unlimited and shall be by Secure Authentication so that these users may access and use Publisher Content in accordance with the terms of this Agreement. Remote access by AuthorisedUsers to Publisher Content is allowed and Walk-In Users, i.e. persons who are permitted by the Customer to access Publisher Content whilst they are on Customer's physical premises. Such access must at all times be by Secure Authentication on computer terminals within the Customer's physical premises. Walk-In Users may not be given means to access Publisher Content outside the Customer's physical premises or by any wireless network unless the network is a secure network provided by the Customer. "Commercial Use" means use which is for direct monetary reward or commercial advantage, whether by or for Customer or Authorised User, by means of the sale, resale, loan, transfer, hire or other form of exploitation of Publisher Content.