Clinical Practice Guideline: Ménière's Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
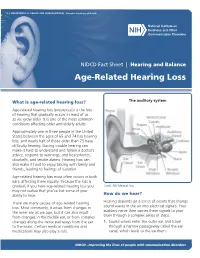
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Press Release
Ménière's Society for dizziness and balance disorders NEWS RELEASE Release date: Monday 17 September 2018 Getting help if your world is in a spin Feeling dizzy and losing your balance is an all too common problem for many people but would you know what to do and where to get help if this happened to you? A loss of balance can lead to falls whatever your age. Around 30% of people under the age of 65 suffer from falls with one in ten people of working age seeking medical advice for vertigo which can lead to life changing or life inhibiting symptoms. Balance disorders are notoriously difficult to diagnose accurately. Most people who feel giddy, dizzy, light-headed, woozy, off-balance, faint or fuzzy, have some kind of balance disorder. This week (16-22 September 2018) is Balance Awareness Week. Organised by the Ménière's Society, the only registered charity in the UK dedicated solely to supporting people with inner ear disorders, events up and down the country will aim to raise awareness of how dizziness and balance problems can affect people and direct them to sources where they can find support. This year Balance Awareness Week also sees the launch of the world’s first Balance Disorder Spectrum, enabling for the first time easy reference to the whole range of balance disorders in one easy to use interactive web site. Medical science is continuously updating and revising its understanding of these often distressing and debilitating conditions. The many different symptoms and causes of balance disorders mean that there have been few attempts to show what they have in common, until now. -

How Do I Know If I Have a Balance Disorder?
PO BOX 13305 · PORTLAND, OR 97213 · FAX: (503) 229-8064 · (800) 837-8428 · [email protected] · WWW.VESTIBULAR.ORG How Do I Know if I Have a Balance Disorder? This article is adapted from information provided by the National Institute on Deafness and Other Communications Disorders, (NIDCD). Millions of individuals have disorders of sensation of spatial disorientation or balance they describe as “dizziness.” imbalance. Experts believe that more than four out of ten Americans will experience an Almost everyone experiences a few episode of dizziness significant enough to seconds of dizziness or disequilibrium at send them to a doctor. some point —for example, when a person stands on a train platform and What can be difficult for both a patient momentarily perceives an illusion of and his or her doctor is that the word moving as a train rushes past. However, “dizziness” is a subjective term. This for some people, symptoms can be means that the word can be used by intense and last a long time, affecting a people to describe different sensations person’s independence, ability to work, they are experiencing, but it is hard for and quality of life. anyone but the person experiencing the symptoms to understand or measure Balance disorders can be caused by the nature or severity of the sensations. medications or certain health conditions, In addition, people tend to use different including problems with the inner ear terms to describe the same kind of (vestibular) organs or the brain. problem. “Dizziness,” “vertigo,” and Dizziness, vertigo, and disequilibrium are “disequilibrium” are often used all symptoms that can result from a interchangeably, even though they have peripheral vestibular disorder (a different meanings. -

A Molecular and Genetic Analysis of Otosclerosis
A molecular and genetic analysis of otosclerosis Joanna Lauren Ziff Submitted for the degree of PhD University College London January 2014 1 Declaration I, Joanna Ziff, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Where work has been conducted by other members of our laboratory, this has been indicated by an appropriate reference. 2 Abstract Otosclerosis is a common form of conductive hearing loss. It is characterised by abnormal bone remodelling within the otic capsule, leading to formation of sclerotic lesions of the temporal bone. Encroachment of these lesions on to the footplate of the stapes in the middle ear leads to stapes fixation and subsequent conductive hearing loss. The hereditary nature of otosclerosis has long been recognised due to its recurrence within families, but its genetic aetiology is yet to be characterised. Although many familial linkage studies and candidate gene association studies to investigate the genetic nature of otosclerosis have been performed in recent years, progress in identifying disease causing genes has been slow. This is largely due to the highly heterogeneous nature of this condition. The research presented in this thesis examines the molecular and genetic basis of otosclerosis using two next generation sequencing technologies; RNA-sequencing and Whole Exome Sequencing. RNA–sequencing has provided human stapes transcriptomes for healthy and diseased stapes, and in combination with pathway analysis has helped identify genes and molecular processes dysregulated in otosclerotic tissue. Whole Exome Sequencing has been employed to investigate rare variants that segregate with otosclerosis in affected families, and has been followed by a variant filtering strategy, which has prioritised genes found to be dysregulated during RNA-sequencing. -
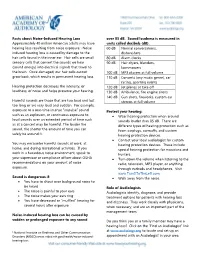
Facts About Noise-Induced Hearing Loss Over 85 Db
Facts about Noise-Induced Hearing Loss over 85 dB. Sound loudness is measured in Approximately 40 million American adults may have units called decibels (dB). hearing loss resulting from noise exposure.1 Noise- 60 dB Normal conversations, induced hearing loss is caused by damage to the dishwashers hair cells found in the inner ear. Hair cells are small 80 dB Alarm clocks sensory cells that convert the sounds we hear 90 dB Hair dryers, blenders, (sound energy) into electrical signals that travel to lawnmowers the brain. Once damaged, our hair cells cannot 100 dB MP3 players at full volume grow back, which results in permanent hearing loss. 110 dB Concerts (any music genre), car racing, sporting events Hearing protection decreases the intensity, or 120 dB Jet planes at take off loudness, of noise and helps preserve your hearing. 130 dB Ambulance, fire engine sirens 140 dB Gun shots, fireworks, custom car Harmful sounds are those that are too loud and last stereos at full volume too long or are very loud and sudden. For example, exposure to a one-time intense “impulse” sound Protect your hearing: such as an explosion, or continuous exposure to • Wear hearing protection when around loud sounds over an extended period of time such sounds louder than 85 dB. There are as at a concert may be harmful. The louder the different types of hearing protection such as sound, the shorter the amount of time you can foam earplugs, earmuffs, and custom safely be around it. hearing protection devices • Contact your local audiologist for custom You may encounter harmful sounds at work, at hearing protection devices. -

Balance Disorder Spectrum Technical Report: January 2018
Balance Disorder Spectrum Technical Report: January 2018 Professor Andrew Hugill Professor Peter Rea College of Science and Engineering The Leicester Balance Centre University of Leicester Department of ENT Leicester, UK Leicester Royal Infirmary [email protected] Leicester, UK Abstract—This paper outlines the technical aspects of the first version of a new spectrum of balance disorders. It describes the II. BALANCE DISORDER SPECTRUM implementation of the spectrum in an interactive web page and proposes some avenues for future research and development. A. Rationale Balance disorders are often both unclear and difficult to Keywords—balance, disorder, spectrum diagnose, so the notion of a spectrum may well prove useful as an introduction to the field. Thanks to existing spectrums (e.g. I. INTRODUCTION autism), the general public is now familiar with the idea of a This project seeks to create an interactive diagnostic tool collection of related disorders ranging across a field that might for the identification of balance disorders, to be used by be anything from mild to severe. The importance of balance clnicians and GPs as well as the wider public. There are three disorders is generally underestimated. The field has suffered main layers to the project: from a fragmented nomenclature and conflicting medical opinions, resulting from a general uncertainty and even • the establishment of the concept of a spectrum of confusion about both symptoms and causes. Terms such as: balance disorders; dizzy, light-headed, floating, woozy, giddy, off-balance, • the creation of an interactive web page providing feeling faint, helpless, or fuzzy, are used loosely and access to the spectrum and its underlying medical interchangeably, consolidating the impression that this is a information; vague collection of ailments. -

Mal De Debarquement Syndrome (Mdds)?
What is Mal de Debarquement Syndrome (MdDS)? MdDS is a rare and life-altering balance disorder that most commonly develops after an ocean cruise or other type of water travel. MdDS is also known as disembarkment disease or persistent landsickness. MdDS also occurs following air/train travel or other motion experiences or spontaneously/in the absence of a motion event. MdDS is a syndrome because it often includes a diverse array of symptoms. The characteristic symptom of MdDS is a persistent sensation of motion such as rocking, swaying and/or bobbing. Other MdDS Symptoms: MAL de • Disequilibrium - a sensation of unsteadiness DEBARQUEMENT or loss of balance • Fatigue; extreme, unusual SYNDROME • Cognitive impairment - difficulty concentrating, confusion, memory loss …a persistent • Anxiety, depression motion and imbalance disorder… • Ataxia – unsteady, staggering gait • Sensitivity to flickering lights, loud or sudden noises, fast or sudden movements, enclosed areas or busy patterns Do you know a person who has • Headaches, including migraine headaches • Heaviness - sensation of gravitational pull of returned from an ocean cruise and the head, body or feet feels like they are still on the boat – • Dizziness months/years later? • Ear pain and/or fullness • Tinnitus – ringing in the ears Perhaps they returned from a • Nausea plane, train or lengthy car ride, and Most MdDS patients feel relief while driving/ it now feels like they are on a ship riding in an auto, airplane, train or other at sea. motion activities. However, the abnormal sensation of motion returns as soon as the They may be suffering from Mal de motion activity is suspended. This is a Debarquement Syndrome (MdDS), helpful feature in the diagnosis of MdDS. -

Consultation Diagnoses and Procedures Billed Among Recent Graduates Practicing General Otolaryngology – Head & Neck Surger
Eskander et al. Journal of Otolaryngology - Head and Neck Surgery (2018) 47:47 https://doi.org/10.1186/s40463-018-0293-8 ORIGINALRESEARCHARTICLE Open Access Consultation diagnoses and procedures billed among recent graduates practicing general otolaryngology – head & neck surgery in Ontario, Canada Antoine Eskander1,2,3* , Paolo Campisi4, Ian J. Witterick5 and David D. Pothier6 Abstract Background: An analysis of the scope of practice of recent Otolaryngology – Head and Neck Surgery (OHNS) graduates working as general otolaryngologists has not been previously performed. As Canadian OHNS residency programs implement competency-based training strategies, this data may be used to align residency curricula with the clinical and surgical practice of recent graduates. Methods: Ontario billing data were used to identify the most common diagnostic and procedure codes used by general otolaryngologists issued a billing number between 2006 and 2012. The codes were categorized by OHNS subspecialty. Practitioners with a narrow range of procedure codes or a high rate of complex procedure codes, were deemed subspecialists and therefore excluded. Results: There were 108 recent graduates in a general practice identified. The most common diagnostic codes assigned to consultation billings were categorized as ‘otology’ (42%), ‘general otolaryngology’ (35%), ‘rhinology’ (17%) and ‘head and neck’ (4%). The most common procedure codes were categorized as ‘general otolaryngology’ (45%), ‘otology’ (23%), ‘head and neck’ (13%) and ‘rhinology’ (9%). The top 5 procedures were nasolaryngoscopy, ear microdebridement, myringotomy with insertion of ventilation tube, tonsillectomy, and turbinate reduction. Although otology encompassed a large proportion of procedures billed, tympanoplasty and mastoidectomy were surprisingly uncommon. Conclusion: This is the first study to analyze the nature of the clinical and surgical cases managed by recent OHNS graduates. -

Balancing Everything out Fifteen Years of Research Into Dizziness, Balance and the Pathology of Ménière’S Disease
Making It Better Balancing Everything Out Fifteen years of research into dizziness, balance and the pathology of Ménière’s disease The Ménière’s Society The Page 1 www.menieres.org.ukMénière’s Society Making It Better Index of References and Sources Further details of the reference sources and research projects discussed in this paper can be found at the following locations: V Osei -Lah, B Ceranic and L M Luxon (2008). Kirby, S.E. and Yardley, L. (2009) Clinical value of tone burst vestibular evoked The contribution of symptoms of post-traumatic myogenic potentials at threshold in acute stress disorder (PTSD), health anxiety and and stable Ménière’s disease. The Journal intolerance of uncertainty to distress in Ménière’s of Laryngology & Otology, 122, pp 452 457 disease. Journal of Nervous and Mental Disease, doi:10.1017/S0022215107009152 197,(5), 324-329 www.journals.cambridge.org/action/ www.eprints.soton.ac.uk/54655 displayAbstract?fromPage=online&aid=1850772 Yardley L., Dibb B. and Osborne G. (2003) A W Morrison, M E S Bailey and G A J Morrison Factors associated with quality of life in Meniere’s (2009). Familial Ménière’s disease: clinical and disease. Clinical Otolaryngology, 28, (5), 436-441. genetic aspects. The Journal of Laryngology (doi:10.1046/j.1365-2273.2003.00740.x). & Otology, 123, pp 29-37. doi:10.1017/S0022215108002788. Yardley L, Kirby S. Evaluation of booklet-based www.journals.cambridge.org/action/ self-management of symptoms in Ménière’s displayAbstract?fromPage=online&aid=3107936 disease: a randomized controlled trial. Psychosom Med 2006;68:762–9 Clinical and cost effectiveness Sandhu, J. -

Hearing Loss
Randal W. Swenson, M.D. Joshua G. Yorgason, M.D. David K. Palmer, M.D. Wesley R. Brown, M.D. John E. Butler, M.D. Nancy J. Stevenson, PA-C Justin D. Gull, M.D. ENT SPECIALISTS Kristin G. Hoopes, PA-C www.entslc.com Hearing Loss Approximately one in ten persons in the United may result from blockage of the ear canal (wax), States has some degree of hearing loss. Hearing is from a perforation (hole) in the ear drum, or from measured in decibels (dB), and a hearing level of 0- infection or disease of any of the three middle ear 25 dB is considered normal hearing. Your level is: bones. With a conductive loss only, the patient will never go deaf, but will always be able to hear, either Right ear _______ dB Left ear _______dB with reconstructive ear surgery or by use of a properly fitted hearing aid. Some patients who are Hearing Severity / % Loss not candidates for surgery, may benefit from a new 25 dB (normal).….0% 65dB(Severe)……...60% technology, the Baha (bone-anchored hearing aid). 35 dB (mild)……..15% 75dB(Severe)……...75% When there is a problem with the inner ear or 45 dB (moderate)..30% >85dB (Profound)..>90% nerve of hearing, a sensori-neural hearing loss occurs. This is most commonly from normal aging, Normal speech discrimination is 88-100%. Yours is: is usually worse in high frequencies, and can progress to total deafness. Noise exposure is another Right ear _______ % Left ear_______% common cause of high frequency hearing loss. Patients with sensori-neural hearing loss usually complain of difficulty hearing in loud environments. -

Secondary Endolymphatic Hydrops
PO BOX 13305 · PORTLAND, OR 97213 · FAX: (503) 229-8064 · (800) 837-8428 · [email protected] · WWW.VESTIBULAR.ORG Secondary Endolymphatic Hydrops By Susan Pesznecker, RN; Legacy Clinical Research & Technology Center, Portland, Oregon with the Vestibular Disorders Association Endolymphatic hydrops is a disorder of controls are believed to be lost or the vestibular system. Although its damaged. This may cause the volume and underlying cause and natural history are concentration of the endolymph to unknown, it is believed to result from fluctuate in response to changes in the abnormalities in the quantity, body’s circulatory fluids and electrolytes. composition, and/or pressure of the endolymph (the fluid within the Symptoms endolymphatic sac, a compartment of the Symptoms typical of hydrops include inner ear). pressure or fullness in the ears (aural fullness), tinnitus (ringing or other noise Causes in the ears), hearing loss, dizziness, and Endolymphatic hydrops may be either imbalance. primary or secondary. Primary idiopathic endolymphatic hydrops (known as Diagnosis and testing Ménière’s disease) occurs for no known There is no vestibular or auditory test reason. Secondary endolymphatic that is diagnostic of endolymphatic hydrops appears to occur in response to hydrops. Diagnosis is clinical—based on an event or underlying condition. For the physician’s observations and on the example, it can follow head trauma or ear patient’s history, symptoms, and surgery, and it can occur with other inner symptom pattern. The clinical diagnosis ear disorders, allergies, or systemic may be strengthened by the results of disorders (such as diabetes or certain tests. For example, certain autoimmune disorders). -

Etiologies and Characteristics of Deaf-Blindness
Etiologies and Characteristics of Deaf-Blindness Kathryn Wolff Heller, R.N., Ph.D. Geor gia State Uni ver sity Cheryl Ken nedy Uni ver sity of Pitts burgh Edited by: Louis Cooper , M.D. Law rence T. Eschelman, M.D., P.C. James W. Long, M.D., P.C. Rosanne K. Silberman, Ed., D. Contents Pref ace··························3 Sec tion I Foun da tions ······················5 Chap ter 1 Def i ni tions and Ter mi nol ogy Per tain ing to Deaf-Blind ness ···················6 Chap ter 2 Nor mal Anatomy of the Eye and Ear ········16 Chap ter 3 Common Disor ders of the Eye and Ear ·······22 Sec tion II Causes of Deaf-Blind ness ···············36 Chap ter 4 He red i tary Syn dromes and Dis or ders········37 Chap ter 5 Con gen i tal In fec tions and Teratogens ········50 Chap ter 6 Prematurity and Small for Gesta tional Age·····65 Chap ter 7 Ad ven ti tious Con di tions ···············68 APPENDIX Ref er ences························75 Pref ace here are sev eral dis or ders, syn dromes, in fec tious dise ases, and ad ven ti tious con - di tions that may re sult in an in di vid ual be ing deaf-blind. As a re sult of these var i - Tous eti ol o gies, an in di vid ual who is deaf-blind may ex hibit a range of vi sion and hear ing losses. Sen sory loss may range from mild im pair ment to to tal loss of the abil ity to see and/or hear.