CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

18Th Livestock Census 2007 of RURAL & URBAN TOTAL DISTRICT
18th Livestock Census 2007 District -- Dehradun S.No. Name Of Block Cattle Exotic and Crossbreed Under 1 to Over 2.5 Years along with their status Total 1 Year 2.5 Year Exotic/Crossbreed Breeding Agriculture Bullock Cart Others Male 1 Chakrata 58 28 2 42 0 0 130 2 Kalsi 64 39 0 23 0 0 126 3 Vikasnagar 601 271 47 585 173 16 1693 4 Sahaspur 854 313 114 453 68 5 1807 5 Raipur 375 212 42 315 24 1 969 6 Doiwala 1037 291 68 219 28 28 1671 Total Rural 2989 1154 273 1637 293 50 6396 1 Chakrata (C.B.) 1 0 0 0 0 0 1 2 Vikasnagar (MB) 16 15 11 17 8 0 67 3 Herbatpur (NP) 19 3 0 4 0 0 26 4 Mussorie (MB) 83 12 1 4 4 9 113 5 Landora (CB) 0 0 0 0 0 0 0 6 Dehradun M.Corp. 240 36 7 6 4 5 298 7 Dehradun (CB) & 8 FRI College Area 18 21 0 0 0 0 39 9 Clement Town (CB) 32 0 0 4 3 0 39 10 Raipur (CT) 51 12 2 8 2 0 75 12 Doiwala (NP) 16 10 0 19 0 2 47 11 Rishikesh (MB) 84 21 2 0 0 0 107 13 I.TS Virbhadra 1 0 0 0 0 0 1 14 Pratitnagar (CT) 16 1 0 0 0 0 17 Total Urban 577 131 23 62 21 16 830 Total District 3566 1285 296 1699 314 66 7226 1 18th Livestock Census 2007 District -- Dehradun S.No. -

Dehradun at a Glance
Dehradun At A Glance Map Of Dehradun Map Of Uttarakhand GENERAL Headquarter Dehradun Relative CMIE Index of Total area 3088.00 sq.km. 142.00 Developement Population Growth per Forest area 2200.56 sq.km. 2.91 annum Population Net sown area 550.57 sq.km. 332.00 density(persons/sq.km) Net Irri. area 217.53 sq.km. Urbanisation 50.26 Occupied Houses 189.30 thousand Literacy 69.50 Total Population 1025.68 thousand Male literacy 77.95 Total Males 556.43 thousand Female literacy 59.26 Total Females 469.25 thousand Urban literacy 81.04 Urban Population 515.48 thousand Rural literacy 57.34 Workers as % of total Urban Pop Male 282.32 thousand 34.54 population Urban Pop 233.16 thousand Agriculture & allied activ. 35.29 Female Rural Population 510.20 thousand Mining & quarrying 0.22 Mfg.(non-household) Rural Pop Male 274.11 thousand 11.34 industries Rural Pop Female 236.09 thousand Households industries 0.86 Total Literates 597.39 thousand Construction workers 4.78 Tot Male Lits 367.11 thousand Other services 47.51 Forest area as % of Tot Fem Lits 230.27 thousand 69.76 reporting area Net sown area as % of Rur Literates 239.97 thousand 17.45 reporting area Gross Irri.area as % of Rur Male Lits 154.96 thousand 38.78 reporting area Average size of operational Rur Fem Lits 85.01 thousand 0.92 holding Fertiliser consumption per Urban Literates 357.42 thousand 46.00 Hect. Value of output of major Urban Male Lits 212.16 thousand 4282.00 crops/hecta Value of output of major Urban Fem Lits 145.26 thousand 357.00 crops/capit Per capita food grains Rur Male Litcy -

ALL INDIA INSTITUTE of MEDICAL SCIENCES Virbhadra Road
ALL INDIA INSTITUTE OF MEDICAL SCIENCES Virbhadra Road, Rishikesh-249203 Uttarakhand Website: www.aiimsrishikesh.edu.in Advertisement No: 21/14/2016(RIS)/ADMN/0783 Dated: 29-04-2017 RECRUITMENT OF NURSING FACULTY POSTS (GROUP A) AT AIIMS RISHIKESH All India Institute of Medical Sciences, Rishikesh an Autonomous Institute of National Importance is one of the new AIIMS and apex healthcare being established by the Ministry of Health & Family Welfare, Government of India under the Pradhan Mantri Swasthya Suraksha Yojna (PMSSY) with the aim of correcting regional imbalance in quality tertiary level healthcare in the country, and attaining self-sufficiency in graduate and postgraduate medical education and training. Online applications are invited for the following faculty posts on DIRECT RECRUITMENT BASIS FOR COLLEGE OF NURSING in All India Institute of Medical Sciences, Rishikesh (Uttarakhand). Sl. No. Posts No of posts 1 Assistant Professor / Lecturer in Nursing 05 (UR-4, OBC-1) Number of vacancies is subject to change (increase / decrease/ cancelled). Director of the institute reserves rights to fill the seats in phased manner AIIMS administration reserves the right to decide the nature of appointment (direct/deputation) to be offered to selected candidates. Reservation policy will be as per Government of India guidelines issued from time to time. I. Application Process: Applications are invited from Indian Nationals in the prescribed form through online mode for faculty posts in AIIMS Rishikesh. The link for online application portal is available at the institute website. The on-line filling up of application form will start on 29.04.2017 (10:00 AM) and will automatically close on 28.06.2017 (05:00 PM). -

TENDER NOTICE for Hospital Furniture AIIMS, Rishikesh, Virbhadra Marg, Rishikesh, Dehradun Date: 21Th June, 2013
Tender Enquiry No. F.No.24/07/2013-RIS (Admin) Cost- Rs.1000 VAT- Rs.135 TENDER NOTICE Total Cost-1135 For Hospital Furniture AIIMS, Rishikesh, Virbhadra Marg, Rishikesh, Dehradun Date: 21th June, 2013 On behalf of the Director, All India Institute of Medical Sciences, Rishikesh tenders in sealed cover are invited under two-bid system from manufacture and their authorised dealers/ distributors for providing Hospital Furniture for AIIMS Rishikesh. The interested manufacture and their authorised dealers/ distributors are required to submit the technical and financial bid separately. The bids in Sealed Cover-I containing “Technical Bid” and Sealed Cover-II containing “Financial Bid” should be placed in a third sealed cover super scribed “Tender For Hospital Furniture” and should reach at the office of “The Administrative Officer, AIIMS, Virbhadra, Marg Rishikesh (Dehradun) - 249201, before 1500 hrs on or before 15/7/ 2013.The bid received after due date and time will not be entertained whatsoever may be the reason. The technical bids shall be opened on the same day at 1700 hrs at AIIMS, Rishikesh. In the event of any of the above mentioned date being declared as a holiday / closed day, the tenders will be opened on the next working day at the appointed time. The financial bid of technically qualified agencies will be open on next working day at 3.30 pm. The tender document containing technical bid form, financial bid form, technical description/specification & item and terms & conditions can be purchased from AIIMS, Rishikesh from 22/6/13 to 15/7/13 between 1000 and 1400 hrs on non-refundable payment of Rs.1135.00 only or can be downloaded from website www.aiimsrishikesh.edu.in. -

Uttarakhand Covid-19 Telephone/Mobile Directory Fueu
fnukad& 27-04-2021 le; 7%00 lka; rd v|ru Uttarakhand Covid-19 Telephone/Mobile Directory Note: ,slk laHko gS fd bl Mk;jsDVjh dks rS;kj djrs le; dfri; =qfV;ka@dfe;k¡ jg xà gks]a ;fn dksà =qfV fdlh ds laKku esa vkrh gS rks —i;k bl Ãesy ij lwfpr djus dk d"V djsa rkfd mls nwj fd;k tk ldsA& [email protected] bl Mk;jsDVjh dks yxkrkj v|ru fd;k tk jgk gS] uohure çfr Mkmuyksm djus ds fy, bl Çyd dks fDyd djsa & https://health.uk.gov.in/files/Covid-19-Directory.pdf fuEu tkudkjh ds fy, fn;s x;s fyad ij fDyd djsa& Sample Collection Centres https://covid19.uk.gov.in/map/sccLocation.aspx Availability of Beds https://covid19.uk.gov.in/bedssummary.aspx Covid-19 Vaccination Sites www.cowin.gov.in RT – PCR Testing Report www.covid19.uk.gov.in To get Medical assistant and http://www.esanjeevaniopd.in/Register Doctor's consultation: Download app: Free Consultation from https://play.google.com/store/apps/details?id=in.hi Esanjeevani ed.esanjeevaniopd&hl=en_US Or call: 9412080703, 9412080622, 9412080686 Home Isolation Registration https://dsclservices.org.in/self-isolation.php Vaccination (Self Registration) www.cowin.gov.in https://selfregistration.cowin.gov.in/ Any other Help Call 104 (24X7) / 0135-2609500/ District Control Room for any other help and assistance. Daily Bulletin https://health.uk.gov.in/pages/display/140-novel- corona-virus-guidelines-and-advisory- Travelling to/from https://dsclservices.org.in/apply.php Uttarakhand Registration for any queries and [email protected] suggestions 104/0135 2609500 1 tuinksa ls lacafèkr tkudkjh ds fy, tuinksa ds uke ij fDyd djsa 1. -
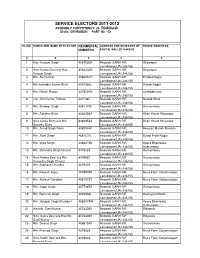
Rishikesh Distic- DEHRADUN PART N0
SERVICE ELECTORS 2011-2012 ASSEMBLY CONSTITENCY- 24- Rishikesh Distic- DEHRADUN PART N0. 154 SL.NO. RANKS AND NAME OF ELECTOR REGIMENTAL ADDRESS FOR DESPTACH OF HOUSE ADDERESS NUMBERS POSTAL BALLOT PAPERS 1 2 3 4 5 1 Hav. Kalyam Singh 4064230N Records GARH RIF, Shyampur Lansdowne(UA)-246155. 2 Smt. Kamla Devi w/o Hav. 4064230N Records GARH RIF, Shyampur Kalyam Singh Lansdowne(UA)-246155. 3 Rfn. Anil Kumar 4083051H Records GARH RIF, Prateet Nagar Lansdowne(UA)-246155. 4 Rfn harendra Kumar Bisht 4081656L Records GARH RIF, Sahab Nagar Lansdowne(UA)-246155. 5 Rfn. Nitesh Rawat 4079200N Records GARH RIF, Chhidderwala Lansdowne(UA)-246155. 6 Lnk. Anil Kumar Tirthwal 4071381 Records GARH RIF, Nawab Wala Lansdowne(UA)-246155. 7 Rfn. Surbeer Singh 4081372F Records GARH RIF, Gumaniwala Lansdowne(UA)-246155. 8 Rfn. Sanjeev Bisht 4080956A Records GARH RIF, Kheri Khurb Shyampur Lansdowne(UA)-246155. 9 Smt. Uimla Bisht w/o Rfn. 4080956A Records GARH RIF, Kheri Khurb Shyampur Sanjeev Bisht Lansdowne(UA)-246155. 10 Rfn. Anind Singh Rana 4080934F Records GARH RIF, Hosiyari Mandir Raiwala Lansdowne(UA)-246155. 11 Rfn. Sunil Singh 4081673L Records GARH RIF, Dandi Pratit Nagar Lansdowne(UA)-246155. 12 Rfn. Vijay Singh 4080279N Records GARH RIF, Kpara Bhaniwala, Lansdowne(UA)-246155. Authunwala 13 Rfn. Surendra Singh Dhanai 4079087 Records GARH RIF, Gumaniwala Lansdowne(UA)-246155. 14 Smt. Rekha Devi w/o Rfn. 4079087 Records GARH RIF, Gumaniwala Surendra Singh Dhanai Lansdowne(UA)-246155. 15 Rfn. Subhash Chandra 4079475 Records GARH RIF, Gumaniwala Lansdowne(UA)-246155. 16 Rfn. Rakesh Rana 4078909K Records GARH RIF, Rusa Farm Satyanarayan Lansdowne(UA)-246155. -

Raipur | Sunday | July 11, 2021
- / A1 3 ! 0 ! 0 0 )+,-).$/01 RNI Regn. No. CHHENG/2012/42718, Postal Reg. No. - RYP DN/34/2013-2015 : '( 6' ))*6;7 :/ - / %"#&' (")*+, !"#$ -9,*/ * - ( ,,/O =(- -/ 2/1< ", ",2-* 1,1- ,3* =* /= 3-/-> ( -2*( , .(- !" 2 $$2"$3 /41@ $ %'( $) ,*-./ -" -+, entire data on the official por- tal. As per WHO guidelines, ithin the next four to six EUL is a procedure to stream- Wweeks, the World Health line the process by which new New Delhi: Highly transmis- Organization (WHO) will or unlicensed products can be sible Delta Plus variant has now decide on including Bharat used during public health reached the Northeastern Biotech’s Covaxin in the emer- emergencies. region of the country also with gency use list (EUL). Sources “There is a process to be Tripura, for the first time, The UP Law Commission said once the decision is taken, followed for EUL and pre- reporting 138 cases. chairman said the new Bill has it will boost the export status of qualification of vaccines under At least 90 per cent of provisions to debar people who the Hyderabad-based pharma which a company has to com- samples in Tripura that were R have more than two children maker and make it eligible for plete phase 3 trials and submit sent for genome sequencing from benefitting Government “green passport.” the whole data to the regulatory have been found to be con- schemes. WHO chief scientist department of WHO which is taining Delta Plus variant, offi- “If somebody doesn’t fol- Soumya Swaminathan has said examined by an expert advi- cial said. low this policy, they won’t be the global body is reviewing sory group,” Swaminathan and Environment (CSE) However, hours after the eligible for such schemes. -

Sign of Bidder TENDER NOTICE AIIMS, Rishikesh, Virbhadra Marg
Page 1 of 30 Tender Enquiry No. F.No.24/Eqpt/15/2013-RIS (Admin) Cost- Rs.1000 TENDER NOTICE Equipments/Instruments for Department of Dentistry VAT- Rs.135 AIIMS, Rishikesh, Virbhadra Marg, Rishikesh, Dehradun Total Cost-1135 Date: ____July,2013 On behalf of the Director, All India Institute of Medical Sciences, Rishikesh tenders in sealed cover are invited under two-bid system from manufacture and their authorised dealers/ distributors for providing Equipments/Instruments for Department of Dentistry for AIIMS Rishikesh. The interested manufactures and their authorised dealers/ distributors are required to submit the technical and financial bid separately. The bids in Sealed Cover-I containing “Technical Bid” and Sealed Cover-II containing “Financial Bid” should be placed in a third sealed cover super scribed “Tender For Equipments/Instruments for Department of Dentistry” and should reach at the office of “The Administrative Officer, AIIMS, Virbhadra, Marg Rishikesh (Dehradun) - 249201, by or before 03.00 PM on 12-08-2013. The bid received after due date and time will not be entertained whatsoever may be the reason. The technical bids shall be opened on the next day i.e 13-08-2013 at 11.00 AM at AIIMS, Rishikesh. In the event of any of the above mentioned date being declared as a holiday / closed day, the tenders will be opened on the next working day at the appointed time. The date of technical evaluation of items and opening of financial bid of technically qualified agencies will be announced later. The tender document containing technical bid form, financial bid form, technical description/specification & item and terms & conditions can be purchased from AIIMS, Rishikesh from 22-07-2013 to 12-08-2013 between 1000 AM and 02.00 PM on non-refundable payment of Rs.1135.00 (Rupees one thousand one hundred thirty five only) or can be downloaded from website www.aiimsrishikesh.edu.in. -

Registered Establishment List in Uttarakhand
S. NoPlace /District Name Of Establishment Address Registration Seasts allocated by Filled Seats Contact No. E-Mail ID No. Establishment 1 Dehradun CONSUMER ELECTRONICS MAINTENANCE CONSUMER ELECTRONICS MAINTENANCE E01160500001 0 0 9837013306 [email protected] COMPANY COMPANY 14/4, BHAGWAN DAS BUILDING, Dehradun 2 Bageshwar Almora Magnesite Limited M/s. Almora Magnesite Limited P.O.- Billori E01160500003 Electrician14, Turner 2, Fitter 2, Electrician 6, Turner 1, Fitter 05963-255012 [email protected] Mechanic (Motor Vehicle) 2, 2,Mechanic (Motor Vehicle) Welder (Gas & Diesel ) 2 2,Welder Gas & Diesel 0 3 Dehradun DEFENCE ELECTRONICS APPLICATION DEFENCE ELECTRONICS APPLICATION E01160500005 Electronics Mechanic10, Fitter4, Electronics Mechanic 9 ,Fitter 1352787062, [email protected], LABORATORY LABORATORY, RAIPUR ROAD, DEHRADUN Machinist 4, Turner 2 4, Machinist 4, Turner 2 01352223105 [email protected] 4 Tehri Garhwal THDC INDIA LIMITED THDC INDIA LIMITED, TOP TERRACE, E01160500006 Gardner2 , Apprenticip Food and Gardner2 , Apprenticip Food 9411103768 [email protected] BHAGIRATHI BHAWAN, BHAGIRATHI PURAM, Beavrage Service4, Apprenticip and Beavrage Service4, Tehri Garhwal Food Production2, House Apprenticip Food Production2, Keeper Hotel1, House Keeper House Keeper Hotel1, House Hospital1 Keeper Hospital1 5 Udham Singh SANSERA ENGINEERING PVT. LTD. SANSERA ENGINEERING PVT. LTD. Plot No. E01160500008 Electrician 10, Fitter 12, Electrician 8 Fitter 5 9997193702, [email protected], Nagar 18, Sector 9, IIE Pantnagar, Distt. Udham Machinist4 9997754510 [email protected] Singh Nagar, Uttarakhand 6 Udham Singh LUMAX INDUSTRIES LTD. LUMAX INDUSTRIES LTD. SEC-11, PLOT NO- E01160500009 Maintenance Mechanic 2, 0 9639551175 [email protected] Nagar 51 IIE SIDCUL PANTNAGAR, Udham Singh Nagar 7 Dehradun Indian Institute of Remote Sensing Indian Institute of Remote Sensing 4, Kalidas E01160500012 0 0 1352524303, [email protected], [email protected] Road, PO Box No. -
Rishikesh(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in Which the Assembly Constituency Is Located: 5-Hardwar(GEN) Electors
ELECTORAL ROLL - 2017 STATE - (S28) UTTARAKHAND No., Name and Reservation Status of Assembly Constituency: 24-Rishikesh(GEN) Last Part No., Name and Reservation Status of Parliamentary Service Constituency in which the Assembly Constituency is located: 5-Hardwar(GEN) Electors 1. DETAILS OF REVISION Year of Revision : 2017 Type of Revision : De-novo preparation Qualifying Date : 01.01.2017 Date of Draft Publication: 04.10.2017 2. SUMMARY OF SERVICE ELECTORS A) NUMBER OF ELECTORS 1. Classified by Type of Service Name of Service No. of Electors Members Wives Total A) Defence Services 999 6 1005 B) Armed Police Force 0 0 0 C) Foreign Service 1 0 1 Total in Part (A+B+C) 1000 6 1006 2. Classified by Type of Roll Roll Type Roll Identification No. of Electors Members Wives Total I Original Preliminary Preliminary De-novo 1000 6 1006 Roll, 2017 preparation of last part of Electoral Roll Net Electors in the Roll 1000 6 1006 Elector Type: M = Member, W = Wife Page 1 Draft Electoral Roll, 2017 of Assembly Constituency 24-Rishikesh (GEN), (S28)UTTARAKHAND A . Defence Services Sl.No Name of Elector Elector Rank Husband's Regimental Address for House Address Type Sl.No. despatch of Ballot Paper (1) (2) (3) (4) (5) (6) (7) Assam Rifles 1 SATYE SINGH M Rifleman Headquarters Directorate General KHADARI KHADAK Assam Rifles, Record Branch, MAP(MOTA PLOT) Laitumkhrah,Shillong-793011 SATYA NARAYAN MANDIR 249204 RISHIKESH 2 DEB BAHADUR M Rifleman Headquarters Directorate General PARTIT NAGAR DANDI CHHETRI Assam Rifles, Record Branch, RAIWALA 000000 Laitumkhrah,Shillong-793011 -
299161603.Pdf
YEAR YEA BATC SR NAME NICK DOB H FROM R TO H ADDRESS ADDRESS1 CITY COUNTRY PHONE PHONE1 MOBILE E-MAIL 1 A. BENERJEE C/O P. BENERJEE TRACTOR LTD. NO. 1, TARATOLLA RODA, GARDEN RD KOLKATA INDIA 2 A. DAVID ANGAMI 1996 1996 WAR CEMETERY KOHIMA NAGALAND INDIA 3 A. J. GEORGE BRO. PRINCIPAL, ST. JOSEPH ACADMEY DEHRADUN INDIA 0135-2712939 0135-2712071 9458131266 [email protected] 4 A. K. JOSHI 1947 1953 1953 NOIDA INDIA 0120-25452053 5 A. M. THANICKEN FSP BRO. 1960 1962 1962 ST. PATRICK, JUNIORATE ADYAAR CHENNAI - 600020 INDIA 6 A. P. S. CHAUHAN PP. SUNITA CHAUHAN DELHI INDIA 0120-6515560 011-26352801 7 A. P. SINGH 1965 1966 1966 MUSSOORIE INDIA 8 A. RAWLINS TEACHER 148, BURDWOOD HIGHWAY, BURDWOOD EAST VICTORIA-3151 AUSTRALIA 9 A. S. BEDI BRIG. TONY 284, SECTOR 11 - A CHANDIGARH INDIA 0172-2747762 10 A. S. PANWAR DR. INDIA 11 A. S. SANDHU LT.GEN. PVSM 15.2.40 1948 1955 1955 F-605 , SOM VIHAAR R.K. PURAM NEW DELHI - 110022 INDIA 0172-2606959 9810447730 12 AAKASH DEEP 2.5.91 C 2001 2009 2009 T/501, BLOCK - B, OM NIRMALAYA APPTS NAGESHWAR COLONY BOUINGR, PATNA 13 ABDULLAH MOHD 1979 ZAKARIA HOUSE, E-37, KALINDI COLONY, NEW DELHI INDIA 9810188034 14 ABDUS SAMAD HASHMI 1998 1998 TAHA SABBAN ESTATE P.O. BOX 9661 JEDDAH SAUDI ARABIA 15 ABEN LAL CHOTA MANGO 29.05.54 M 1963 1969 1969 FLAT NO.- C-8/8292 VASANT KUNJ, NEW DELHI INDIA 011-25672261 011-26133599 9810997722 [email protected] 16 ABHAY JIT JOSHI 1.12.92 C 2001 2009 2009 15/ 13, SUBHASH NAGAR HALDWANI UTRANCHAL INDIA 05942-237227 9412968275 [email protected] 17 ABHAY KUMAR M 1994 2002 2002 OPP, MANGLAM ENCLAVE MORE PO. -

District Census Handbook District, Deharadun, Part XII-B, Series-25
CENSUS 1991 )}f~c>11-25 SERIES-25 \3ct1x m UTTAR PRADESH 1=fT1T- XII "ij PART-XllB "/II~ q ~~I;fjll VILLAGE & TOWNWISE m~fJi¢ \Ji;:PI UI~I PRIMARY CENSUS ABSTRACT "f.fR ftic>11 \J1~JIUHI gfdgffitCf51 DISTRICT CENSUS HANDBOOK itrc>n ~5xl~'i DISTRICT DEHARADUN f.1~~ICfj \it·-PIOI'""l1 cnm DIRECTOR OF CENSUS OPERATIONS ~~ UTTAR PRADESH 1 !II fcllq;:11 2 >Il'CfcP~ V 3 ~ em &1l'1fi1'3l 4 ~ ~ &16Ci1,!\uf ~ IX 5 ~ \lI~IIOI~1 6«1~R:c1fm ~ ~ ~ ~ ~ 6 fCp~ cl lSI UII c+1 Cf) f?:a:roft XVI 7 ~ ~ \lI~IIUI""I1 X1R 8 lJTlftur /~ ~ \lI""IIIUI~1 X1R 14 31'- (i) lJTlftur 111""1fi1'3l ~ ~ \lI""IJIUI~1 mx tll!!~ I RtCf) fcrq;-m ~ 'qCf):W'I1 22 2 tll!!~I~Cf) fcrq;-m ~-~ 44 3 tll!!~ I ~ Cf) fcrq;-m ~-fcrq;-m "I'"'R 72 4 tll!!~ I ~ Cf) fcrq;-m ~ tl6tl~'< 82 5 tll!!~IRtCf) fcrq;-m ~-~ 98 6 tll!!~ I ~Cf) fcrq;-m ~ '51'~ ql<'11 116 7 ~ 126 (ii) m ~ qUlfjwl1 ~ 1 tll!!~I~Cf) fctct>m ~-'qCf)'<It11 130 2 tll!!~I~Cf) fcrq;-m ~-~ 137 3 tll j~ IRt Cf) fctct>m ~-fctct>m "I'"'R 146 4 '(11!iGI~q; fctcpm ~ '(1 6'(1 ~'( 149 5 '(11!iGI~q; fctcpm ~-~ 155 6 '(11!iGI~q; fctcpm ~ -SltqlC'l1 161 i{- ~ 165 ~ ~ \il'1~IOI"'1I·~ (cni CJR) 6'<EI'(~'( irO-qO II ~ ~ \i1"1~lol'1l ~ (cni CJR) -sltql<.>fl irO-qO III ~ ~ \ij=i~I01"'11 "'(1'R (crrt cnx) fcIcpm w:rR ~O 'tITO IV ~ ~ \l1'1~lol'1l ~ (cni CJR) ~ ~ (\1Ffo "'17R) V ~ ~ \l1'1~lol'1l ~ (crrt CJR) ilCf)'(ldi (k) VI ~ ~ \ij'1~lol'1l ~ (crrt CJR) '(14qlC'l1 (\iRo ~) VII ~ ~ \l1'1~lol'1l ~ (crrt erR) *~~~I(;ro 'tITo) VIII ~ ~ \l1'1~lol'1l "ffiX (crrt erR) ~ (-q;rO ~o) IX ~ ~ \l1'1~lol'1l "ffiX (~ erR) *~~~I (\1Ffo 'llR) X ~ ~ \l1'1~lol'1l