Pholiotina Cyanopus, a Rare Fungus Producing Psychoactive Tryptamines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Occurrence of Psilocybin/Psilocin in Pluteus Salicinus (Pluteaceae)
College of Saint Benedict and Saint John's University DigitalCommons@CSB/SJU Biology Faculty Publications Biology 7-1981 Occurrence of psilocybin/psilocin in Pluteus salicinus (Pluteaceae) Stephen G. Saupe College of Saint Benedict/Saint John's University, [email protected] Follow this and additional works at: https://digitalcommons.csbsju.edu/biology_pubs Part of the Biology Commons, Botany Commons, and the Fungi Commons Recommended Citation Saupe SG. 1981. Occurrence of psilocybin/psilocin in Pluteus salicinus (Pluteaceae). Mycologia 73(4): 781-784. Copyright © 1981 Mycological Society of America. OCCURRENCE OF PSILOCYBIN/ PSILOCIN IN PLUTEUS SALICINUS (PLUTEACEAE) STEPHEN G. SAUPE Department of Botany, University of Illinois, Urbana, Illinois 61801 The development of blue color in a basidiocarp after bruising is a reliable, although not infallible, field character for detecting the pres ence of the N-methylated tryptamines, psilocybin and psilocin (1, 2, 8). This color results from the stepwise oxidation of psilocybin to psi locin to a blue pigment (3). Pluteus salicinus (Pers. ex Fr.) Kummer (Pluteaceae) has a grey pileus with erect to depressed, blackish, spinu lose squamules in the center. It is distinguished from other species in section Pluteus by its bluish to olive-green stipe, the color intensify ing with age and bruising (10, 11 ). This study was initiated to deter mine if the bluing phenomenon exhibited by this fungus is due to the presence of psilocybin/psilocin. Pluteus salicinus (sgs-230, ILL) was collected on decaying wood in Brownfield Woods, Urbana, Illinois, a mixed mesophytic upland forest. Carpophores were solitary and uncommon. Although Singer (10) reponed that this fungus is common in some areas of North America and Europe, it is rare in Michigan (5). -

Diversity of Species of the Genus Conocybe (Bolbitiaceae, Agaricales) Collected on Dung from Punjab, India
Mycosphere 6(1): 19–42(2015) ISSN 2077 7019 www.mycosphere.org Article Mycosphere Copyright © 2015 Online Edition Doi 10.5943/mycosphere/6/1/4 Diversity of species of the genus Conocybe (Bolbitiaceae, Agaricales) collected on dung from Punjab, India Amandeep K1*, Atri NS2 and Munruchi K2 1Desh Bhagat College of Education, Bardwal-Dhuri-148024, Punjab, India 2Department of Botany, Punjabi University, Patiala-147002, Punjab, India. Amandeep K, Atri NS, Munruchi K 2015 – Diversity of species of the genus Conocybe (Bolbitiaceae, Agaricales) collected on dung from Punjab, India. Mycosphere 6(1), 19–42, Doi 10.5943/mycosphere/6/1/4 Abstract A study of diversity of coprophilous species of Conocybe was carried out in Punjab state of India during the years 2007 to 2011. This research paper represents 22 collections belonging to 16 Conocybe species growing on five diverse dung types. The species include Conocybe albipes, C. apala, C. brachypodii, C. crispa, C. fuscimarginata, C. lenticulospora, C. leucopus, C. magnicapitata, C. microrrhiza var. coprophila var. nov., C. moseri, C. rickenii, C. subpubescens, C. subxerophytica var. subxerophytica, C. subxerophytica var. brunnea, C. uralensis and C. velutipes. For all these taxa, dung types on which they were found growing are mentioned and their distinctive characters are described and compared with similar taxa along with a key for their identification. The taxonomy of ten taxa is discussed along with the drawings of morphological and anatomical features. Conocybe microrrhiza var. coprophila is proposed as a new variety. As many as six taxa, namely C. albipes, C. fuscimarginata, C. lenticulospora, C. leucopus, C. moseri and C. -

Major Clades of Agaricales: a Multilocus Phylogenetic Overview
Mycologia, 98(6), 2006, pp. 982–995. # 2006 by The Mycological Society of America, Lawrence, KS 66044-8897 Major clades of Agaricales: a multilocus phylogenetic overview P. Brandon Matheny1 Duur K. Aanen Judd M. Curtis Laboratory of Genetics, Arboretumlaan 4, 6703 BD, Biology Department, Clark University, 950 Main Street, Wageningen, The Netherlands Worcester, Massachusetts, 01610 Matthew DeNitis Vale´rie Hofstetter 127 Harrington Way, Worcester, Massachusetts 01604 Department of Biology, Box 90338, Duke University, Durham, North Carolina 27708 Graciela M. Daniele Instituto Multidisciplinario de Biologı´a Vegetal, M. Catherine Aime CONICET-Universidad Nacional de Co´rdoba, Casilla USDA-ARS, Systematic Botany and Mycology de Correo 495, 5000 Co´rdoba, Argentina Laboratory, Room 304, Building 011A, 10300 Baltimore Avenue, Beltsville, Maryland 20705-2350 Dennis E. Desjardin Department of Biology, San Francisco State University, Jean-Marc Moncalvo San Francisco, California 94132 Centre for Biodiversity and Conservation Biology, Royal Ontario Museum and Department of Botany, University Bradley R. Kropp of Toronto, Toronto, Ontario, M5S 2C6 Canada Department of Biology, Utah State University, Logan, Utah 84322 Zai-Wei Ge Zhu-Liang Yang Lorelei L. Norvell Kunming Institute of Botany, Chinese Academy of Pacific Northwest Mycology Service, 6720 NW Skyline Sciences, Kunming 650204, P.R. China Boulevard, Portland, Oregon 97229-1309 Jason C. Slot Andrew Parker Biology Department, Clark University, 950 Main Street, 127 Raven Way, Metaline Falls, Washington 99153- Worcester, Massachusetts, 01609 9720 Joseph F. Ammirati Else C. Vellinga University of Washington, Biology Department, Box Department of Plant and Microbial Biology, 111 355325, Seattle, Washington 98195 Koshland Hall, University of California, Berkeley, California 94720-3102 Timothy J. -

INTRODUCTION Biodiversity of Agaricomycetes Basidiomes
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CONICET Digital DARWINIANA, nueva serie 1(1): 67-75. 2013 Versión final, efectivamente publicada el 31 de julio de 2013 ISSN 0011-6793 impresa - ISSN 1850-1699 en línea BIODIVERSITY OF AGARICOMYCETES BASIDIOMES ASSOCIATED TO SALIX AND POPULUS (SALICACEAE) PLANTATIONS Gonzalo M. Romano1, Javier A. Calcagno2 & Bernardo E. Lechner1 1Laboratorio de Micología, Fitopatología y Liquenología, Departamento de Biodiversidad y Biología Experimental, Programa de Plantas Medicinales y Programa de Hongos que Intervienen en la Degradación Biológica (CONICET), Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Intendente Güiraldes 2160, Pabellón II, Piso 4, Laboratorio 7, C1428EGA Ciudad Autónoma de Buenos Aires, Argentina; [email protected] (author for correspondence). 2Centro de Estudios Biomédicos, Biotecnológicos, Ambientales y de Diagnóstico - Departamento de Ciencias Natu- rales y Antropológicas, Instituto Superior de Investigaciones, Hidalgo 775, C1405BCK Ciudad Autónoma de Buenos Aires, Argentina. Abstract. Romano, G. M.; J. A. Calcagno & B. E. Lechner. 2013. Biodiversity of Agaricomycetes basidiomes asso- ciated to Salix and Populus (Salicaceae) plantations. Darwiniana, nueva serie 1(1): 67-75. Although plantations have an artificial origin, they modify environmental conditions that can alter native fungi diversity. The effects of forest management practices on a plantation of willow (Salix) and poplar (Populus) over Agaricomycetes basidiomes biodiversity were studied for one year in an island located in Paraná Delta, Argentina. Dry weight and number of basidiomes were measured. We found 28 species belonging to Agaricomycetes: 26 species of Agaricales, one species of Polyporales and one species of Russulales. -

Agarics-Stature-Types.Pdf
Gilled Mushroom Genera of Chicago Region, by stature type and spore print color. Patrick Leacock – June 2016 Pale spores = white, buff, cream, pale green to Pinkish spores Brown spores = orange, Dark spores = dark olive, pale lilac, pale pink, yellow to pale = salmon, yellowish brown, rust purplish brown, orange pinkish brown brown, cinnamon, clay chocolate brown, Stature Type brown smoky, black Amanitoid Amanita [Agaricus] Vaginatoid Amanita Volvariella, [Agaricus, Coprinus+] Volvopluteus Lepiotoid Amanita, Lepiota+, Limacella Agaricus, Coprinus+ Pluteotoid [Amanita, Lepiota+] Limacella Pluteus, Bolbitius [Agaricus], Coprinus+ [Volvariella] Armillarioid [Amanita], Armillaria, Hygrophorus, Limacella, Agrocybe, Cortinarius, Coprinus+, Hypholoma, Neolentinus, Pleurotus, Tricholoma Cyclocybe, Gymnopilus Lacrymaria, Stropharia Hebeloma, Hemipholiota, Hemistropharia, Inocybe, Pholiota Tricholomatoid Clitocybe, Hygrophorus, Laccaria, Lactarius, Entoloma Cortinarius, Hebeloma, Lyophyllum, Megacollybia, Melanoleuca, Inocybe, Pholiota Russula, Tricholoma, Tricholomopsis Naucorioid Clitocybe, Hygrophorus, Hypsizygus, Laccaria, Entoloma Agrocybe, Cortinarius, Hypholoma Lactarius, Rhodocollybia, Rugosomyces, Hebeloma, Gymnopilus, Russula, Tricholoma Pholiota, Simocybe Clitocyboid Ampulloclitocybe, Armillaria, Cantharellus, Clitopilus Paxillus, [Pholiota], Clitocybe, Hygrophoropsis, Hygrophorus, Phylloporus, Tapinella Laccaria, Lactarius, Lactifluus, Lentinus, Leucopaxillus, Lyophyllum, Omphalotus, Panus, Russula Galerinoid Galerina, Pholiotina, Coprinus+, -

Hans Halbwachs
Hans Halbwachs hat are fungal characteristics Moreover, the variability of spore traits in winter. It has been speculated that good for? Well, for identifying is bewildering (as was discussed by Else this may be a strategy to avoid predators fungi, of course! Field Vellinga in the previous issue of FUNGI). (Halbwachs et al., 2016), though this Wmycologists all over the world are living Size, ornamentation, and pigmentation would imply investment, e.g., into encyclopedias when it comes to fungal occur in all combinations (Fig. 2). These antifreeze substances and producing traits. Even the most subtle differences are the visible characteristics which may relatively small fruit bodies, as in in spore size or cap coloration have their be grouped in (1) morphological (shape, Flammulina velutipes or Hygrophorus place in identifying mushrooms and size) and (2) physiological (pigments, hypothejus. Generally, species fruiting other fungi. Quite many mycologists are taste, smell, toxicity, etc.). A third, more in late autumn seem to have larger intrigued by the endless variations, for mysterious trait, is the phenology of fruit fruit bodies, at least in Cortinarius instance of fruit bodies (Fig. 1). bodies. Some do it in spring, some even (Halbwachs, 2018). Figure 1. Examples of basidiomycete fruit body shapes and colors. From left to right top: Amanita flavoconia (courtesy J. Veitch), Lactarius indigo (courtesy A. Rockefeller), Butyriboletus frostii (courtesy D. Molter); bottom: Calostoma cinnabarinum (courtesy D. Molter), Tricholomopsis decora (courtesy W. Sturgeon), Mycena adonis (courtesy D. Molter). creativecommons. org/licenses/by-sa/3.0/deed.en. 18 FUNGI Volume 12:1 Spring 2019 Knockin’ on Evolution’s Door Although some ideas are circulating about the functionality of fungal traits, mycologists want to know more about their ecological implications. -

Amanita Muscaria (Fly Agaric)
J R Coll Physicians Edinb 2018; 48: 85–91 | doi: 10.4997/JRCPE.2018.119 PAPER Amanita muscaria (fly agaric): from a shamanistic hallucinogen to the search for acetylcholine HistoryMR Lee1, E Dukan2, I Milne3 & Humanities The mushroom Amanita muscaria (fly agaric) is widely distributed Correspondence to: throughout continental Europe and the UK. Its common name suggests MR Lee Abstract that it had been used to kill flies, until superseded by arsenic. The bioactive 112 Polwarth Terrace compounds occurring in the mushroom remained a mystery for long Merchiston periods of time, but eventually four hallucinogens were isolated from the Edinburgh EH11 1NN fungus: muscarine, muscimol, muscazone and ibotenic acid. UK The shamans of Eastern Siberia used the mushroom as an inebriant and a hallucinogen. In 1912, Henry Dale suggested that muscarine (or a closely related substance) was the transmitter at the parasympathetic nerve endings, where it would produce lacrimation, salivation, sweating, bronchoconstriction and increased intestinal motility. He and Otto Loewi eventually isolated the transmitter and showed that it was not muscarine but acetylcholine. The receptor is now known variously as cholinergic or muscarinic. From this basic knowledge, drugs such as pilocarpine (cholinergic) and ipratropium (anticholinergic) have been shown to be of value in glaucoma and diseases of the lungs, respectively. Keywords acetylcholine, atropine, choline, Dale, hyoscine, ipratropium, Loewi, muscarine, pilocarpine, physostigmine Declaration of interests No conflicts of interest declared Introduction recorded by the Swedish-American ethnologist Waldemar Jochelson, who lived with the tribes in the early part of the Amanita muscaria is probably the most easily recognised 20th century. His version of the tale reads as follows: mushroom in the British Isles with its scarlet cap spotted 1 with conical white fl eecy scales. -

Near the Himalayas, from Kashmir to Sikkim, at Altitudes the Catholic Inquisition, and the Traditional Use of These of up to 2700 Meters
Year of edition: 2018 Authors of the text: Marc Aixalà & José Carlos Bouso Edition: Alex Verdaguer | Genís Oña | Kiko Castellanos Illustrations: Alba Teixidor EU Project: New Approaches in Harm Reduction Policies and Practices (NAHRPP) Special thanks to collaborators Alejandro Ponce (in Peyote report) and Eduardo Carchedi (in Kambó report). TECHNICAL REPORT ON PSYCHOACTIVE ETHNOBOTANICALS Volumes I - II - III ICEERS International Center for Ethnobotanical Education Research and Service INDEX SALVIA DIVINORUM 7 AMANITA MUSCARIA 13 DATURA STRAMONIUM 19 KRATOM 23 PEYOTE 29 BUFO ALVARIUS 37 PSILOCYBIN MUSHROOMS 43 IPOMOEA VIOLACEA 51 AYAHUASCA 57 IBOGA 67 KAMBÓ 73 SAN PEDRO 79 6 SALVIA DIVINORUM SALVIA DIVINORUM The effects of the Hierba Pastora have been used by Mazatec Indians since ancient times to treat diseases and for divinatory purposes. The psychoactive compound Salvia divinorum contains, Salvinorin A, is the most potent naturally occurring psychoactive substance known. BASIC INFO Ska Pastora has been used in divination and healing Salvia divinorum is a perennial plant native to the Maza- rituals, similar to psilocybin mushrooms. Maria Sabina tec areas of the Sierra Madre Oriental Mountains of Mexi- told Wasson and Hofmann (the discoverers of its Mazatec co. Its habitat is tropical forests, where it grows between usage) that Salvia divinorum was used in times when the- 300 and 800 meters above sea level. It belongs to the re was a shortage of mushrooms. Some sources that have Lamiaceae family, and is mainly reproduced by cuttings done later feldwork point out that the use of S. divinorum since it rarely produces seeds. may be more widespread than originally believed, even in times when mushrooms were abundant. -

Three New Species of Cortinarius Subgenus Telamonia (Cortinariaceae, Agaricales) from China
A peer-reviewed open-access journal MycoKeys 69: 91–109 (2020) Three new species in Cortinarius from China 91 doi: 10.3897/mycokeys.69.49437 RESEARCH ARTICLE MycoKeys http://mycokeys.pensoft.net Launched to accelerate biodiversity research Three new species of Cortinarius subgenus Telamonia (Cortinariaceae, Agaricales) from China Meng-Le Xie1,2, Tie-Zheng Wei3, Yong-Ping Fu2, Dan Li2, Liang-Liang Qi4, Peng-Jie Xing2, Guo-Hui Cheng5,2, Rui-Qing Ji2, Yu Li2,1 1 Life Science College, Northeast Normal University, Changchun 130024, China 2 Engineering Research Cen- ter of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University, Changchun 130118, China 3 State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China 4 Microbiology Research Institute, Guangxi Academy of Agriculture Sciences, Nanning, 530007, China 5 College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, China Corresponding authors: Rui-Qing Ji ([email protected]), Yu Li ([email protected]) Academic editor: O. Raspé | Received 16 December 2019 | Accepted 23 June 2020 | Published 14 July 2020 Citation: Xie M-L, Wei T-Z, Fu Y-P, Li D, Qi L-L, Xing P-J, Cheng G-H, Ji R-Q, Li Y (2020) Three new species of Cortinarius subgenus Telamonia (Cortinariaceae, Agaricales) from China. MycoKeys 69: 91–109. https://doi. org/10.3897/mycokeys.69.49437 Abstract Cortinarius is an important ectomycorrhizal genus that forms a symbiotic relationship with certain trees, shrubs and herbs. Recently, we began studying Cortinarius in China and here we describe three new spe- cies of Cortinarius subg. Telamonia based on morphological and ecological characteristics, together with phylogenetic analyses. -
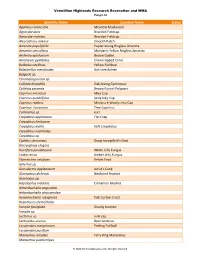
Scientific Name Common Name Status Agaricus Campestris
Vermillion Highlands Research Recreation and WMA Fungi List Scientific Name Common Name Status Agaricus campestris Meadow Mushroom Agrocybe dura Bearded Fieldcap Agrocybe molesta Bearded Fieldcap Aleurodiscus oakesii Smooth Patch Amanita populiphila Poplar-loving Ringless Amanita Amanita sinicoflava Mandarin Yellow Ringless Amanita Arrhenia epichysium Brown Goblet Artomyces pyxidatus Crown-tipped Coral Bolbitius vitellinus Yellow Fieldcap Boletinellus merulioides Ash-tree Bolete Bulgaria sp. Chromelosporium sp. Collybia dryophila Oak-loving Gymnopus Coltricia perennis Brown Funnel Polypore Coprinus micaceus Mica Cap Coprinus quadrifidus Scaly Inky Cap Coprinus radians Miniature Woolly Inky Cap Coprinus truncorum Tree Coprinus Cortinarius sp. cort Crepidotus applanatus Flat Crep Crepidotus herbarum Crepidotus mollis Soft Crepidotus Crepidotus nephrodes Crepidotus sp. Cyathus stercoreus Dung-loving Bird’s Nest Dacryopinax elegans Ductifera pululahuana White Jelly Fungus Exidia recisa Amber Jelly Fungus Flammulina velutipes Velvet Foot Galerina sp. Ganoderma applanatum Artist’s Conk Gloeoporus dichrous Bicolored Bracket Gymnopus sp. Hapalopilus nidulans Cinnamon Bracket Hohenbuehelia angustata Hohenbuehelia atrocaerulea Hymenochaete rubiginosa Oak Curtain Crust Hypomyces tremellicola Inocybe fastigiata Deadly Inocybe Inocybe sp. Lactarius sp. milk cap Lentinellus ursinus Bear Lentinus Lycoperdon marginatum Peeling Puffball Lycoperdon pusillum Marasmius oreades Fairy Ring Marasmius Marasmius pulcherripes © 2020 MinnesotaSeasons.com. All rights -

Wood Chip Fungi: Agrocybe Putaminum in the San Francisco Bay Area
Wood Chip Fungi: Agrocybe putaminum in the San Francisco Bay Area Else C. Vellinga Department of Plant and Microbial Biology, 111 Koshland Hall, Berkeley CA 94720-3102 [email protected] Abstract Agrocybe putaminum was found growing on wood chips in central coastal California; this appears to be the first record for North America. A short description of the species is given. Its habitat plus the characteristics of wood chip denizens are discussed. Wood chips are the fast food of the fungal world. The desir- able wood is exposed, there is a lot of it, and often the supply is replenished regularly. It is an especially good habitat for mush- room species that like it hot because a thick layer of wood chips is warmed relative to the surrounding environment by the activity of bacteria and microscopic fungi (Brown, 2003; Van den Berg and Vellinga, 1998). Thirty years ago wood chips were a rarity, but nowadays they are widely used in landscaping and gardening. A good layer of chips prevents weeds from germinating and taking over, which means less maintenance and lower costs. Chips also diminish evaporation and keep moisture in the soil. Trees and shrubs are often shredded and dumped locally, but there is also long-dis- tance transport of these little tidbits. Barges full of wood mulch cruise the Mississippi River, and trucks carry the mulch from city to city. This fast food sustains a steady stream of wood chip fungi that, as soon as they are established, fruit in large flushes and are suddenly everywhere. The fungi behave a bit like morels after a Figure 1. -

Bibliotheksliste-Aarau-Dezember 2016
Bibliotheksverzeichnis VSVP + Nur im Leesesaal verfügbar, * Dissert. Signatur Autor Titel Jahrgang AKB Myc 1 Ricken Vademecum für Pilzfreunde. 2. Auflage 1920 2 Gramberg Pilze der Heimat 2 Bände 1921 3 Michael Führer für Pilzfreunde, Ausgabe B, 3 Bände 1917 3 b Michael / Schulz Führer für Pilzfreunde. 3 Bände 1927 3 Michael Führer für Pilzfreunde. 3 Bände 1918-1919 4 Dumée Nouvel atlas de poche des champignons. 2 Bände 1921 5 Maublanc Les champignons comestibles et vénéneux. 2 Bände 1926-1927 6 Negri Atlante dei principali funghi comestibili e velenosi 1908 7 Jacottet Les champignons dans la nature 1925 8 Hahn Der Pilzsammler 1903 9 Rolland Atlas des champignons de France, Suisse et Belgique 1910 10 Crawshay The spore ornamentation of the Russulas 1930 11 Cooke Handbook of British fungi. Vol. 1,2. 1871 12/ 1,1 Winter Die Pilze Deutschlands, Oesterreichs und der Schweiz.1. 1884 12/ 1,5 Fischer, E. Die Pilze Deutschlands, Oesterreichs und der Schweiz. Abt. 5 1897 13 Migula Kryptogamenflora von Deutschland, Oesterreich und der Schweiz 1913 14 Secretan Mycographie suisse. 3 vol. 1833 15 Bourdot / Galzin Hymenomycètes de France (doppelt) 1927 16 Bigeard / Guillemin Flore des champignons supérieurs de France. 2 Bände. 1913 17 Wuensche Die Pilze. Anleitung zur Kenntnis derselben 1877 18 Lenz Die nützlichen und schädlichen Schwämme 1840 19 Constantin / Dufour Nouvelle flore des champignons de France 1921 20 Ricken Die Blätterpilze Deutschlands und der angr. Länder. 2 Bände 1915 21 Constantin / Dufour Petite flore des champignons comestibles et vénéneux 1895 22 Quélet Les champignons du Jura et des Vosges. P.1-3+Suppl.