MED-Project Stewardship Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Draft Copy « License Modernization «
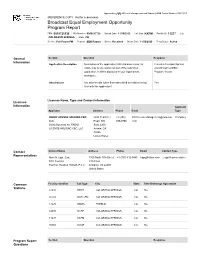
Approved by OMB (Office of Management and Budget) | OMB Control Number 3060-0113 (REFERENCE COPY - Not for submission) Broadcast Equal Employment Opportunity Program Report FRN: 0019721638 File Number: 0000127728 Submit Date: 11/30/2020 Call Sign: KKFM Facility ID: 11237 City: COLORADO SPRINGS State: CO Service: Full Power FM Purpose: EEO Report Status: Received Status Date: 11/30/2020 Filing Status: Active General Section Question Response Information Application Description Description of the application (255 characters max.) is Cumulus-Colorado Springs visible only to you and is not part of the submitted 2020 Broadcast EEO application. It will be displayed in your Applications Program Report workspace. Attachments Are attachments (other than associated schedules) being Yes filed with this application? Licensee Name, Type and Contact Information Licensee Information Applicant Applicant Address Phone Email Type RADIO LICENSE HOLDING CBC, 3280 Peachtree +1 (404) FCCLicenseManagement@cumulus. Company LLC Road, NW 949-0700 com Doing Business As: RADIO Suite 2200 LICENSE HOLDING CBC, LLC Atlanta, GA 30305 United States Contact Contact Name Address Phone Email Contact Type Representatives Mark N. Lipp , Esq . 1300 North 17th Street +1 (703) 812-0445 [email protected] Legal Representative FCC Counsel 11th Floor Fletcher, Heald & Hildreth, P.L.C. Arlington, VA 22209 United States Common Facility Identifier Call Sign City State Time Brokerage Agreement Stations 62038 KKPK COLORADO SPRINGS CO No 66249 KATC-FM COLORADO SPRINGS CO No 11229 KKMG PUEBLO -

530 CIAO BRAMPTON on ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb
frequency callsign city format identification slogan latitude longitude last change in listing kHz d m s d m s (yy-mmm) 530 CIAO BRAMPTON ON ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb 540 CBKO COAL HARBOUR BC VARIETY CBC RADIO ONE N50 36 4 W127 34 23 09-May 540 CBXQ # UCLUELET BC VARIETY CBC RADIO ONE N48 56 44 W125 33 7 16-Oct 540 CBYW WELLS BC VARIETY CBC RADIO ONE N53 6 25 W121 32 46 09-May 540 CBT GRAND FALLS NL VARIETY CBC RADIO ONE N48 57 3 W055 37 34 00-Jul 540 CBMM # SENNETERRE QC VARIETY CBC RADIO ONE N48 22 42 W077 13 28 18-Feb 540 CBK REGINA SK VARIETY CBC RADIO ONE N51 40 48 W105 26 49 00-Jul 540 WASG DAPHNE AL BLK GSPL/RELIGION N30 44 44 W088 5 40 17-Sep 540 KRXA CARMEL VALLEY CA SPANISH RELIGION EL SEMBRADOR RADIO N36 39 36 W121 32 29 14-Aug 540 KVIP REDDING CA RELIGION SRN VERY INSPIRING N40 37 25 W122 16 49 09-Dec 540 WFLF PINE HILLS FL TALK FOX NEWSRADIO 93.1 N28 22 52 W081 47 31 18-Oct 540 WDAK COLUMBUS GA NEWS/TALK FOX NEWSRADIO 540 N32 25 58 W084 57 2 13-Dec 540 KWMT FORT DODGE IA C&W FOX TRUE COUNTRY N42 29 45 W094 12 27 13-Dec 540 KMLB MONROE LA NEWS/TALK/SPORTS ABC NEWSTALK 105.7&540 N32 32 36 W092 10 45 19-Jan 540 WGOP POCOMOKE CITY MD EZL/OLDIES N38 3 11 W075 34 11 18-Oct 540 WXYG SAUK RAPIDS MN CLASSIC ROCK THE GOAT N45 36 18 W094 8 21 17-May 540 KNMX LAS VEGAS NM SPANISH VARIETY NBC K NEW MEXICO N35 34 25 W105 10 17 13-Nov 540 WBWD ISLIP NY SOUTH ASIAN BOLLY 540 N40 45 4 W073 12 52 18-Dec 540 WRGC SYLVA NC VARIETY NBC THE RIVER N35 23 35 W083 11 38 18-Jun 540 WETC # WENDELL-ZEBULON NC RELIGION EWTN DEVINE MERCY R. -

Order and Consent Decree
Federal Communications Commission DA 16-3 Before the Federal Communications Commission Washington, DC 20554 In the Matter of ) ) File No.: EB-IHD-14-000151152 Radio License Holding CBC, LLC ) Acct. No.: 201632080003 ) FRN: 0019721638 Former Licensee of Station WOKQ(FM), ) Facility ID No.: 22887 Dover, New Hampshire1; and ) ) Cumulus Radio Corporation ) FRN: 0001595214 ) ORDER Adopted: January 7, 2016 Released: January 7, 2016 By the Chief, Enforcement Bureau: 1. The Enforcement Bureau (Bureau) of the Federal Communications Commission (Commission) has entered into a Consent Decree to resolve its investigation into whether Radio License Holding CBC, LLC (Radio License), and Radio License’s parent, Cumulus Radio Corporation (CRC), broadcast announcements on radio station WOKQ(FM), Dover, New Hampshire (Station), without adequate sponsorship disclosure in violation of the Commission’s sponsorship identification laws. 2. The Commission’s sponsorship identification laws protect consumers and promote fair competition by requiring that the sponsors of paid programming material be clearly identified. Those laws are based on the principle that listeners and viewers are entitled to know who seeks to persuade them. The disclosures required by those laws provide listeners and viewers with information concerning the source of material in order to prevent misleading or deceiving those listeners and viewers. Enforcement of the sponsorship identification laws also protects fair competition among advertisers. We seek to prevent sponsors from gaining unfair advantage by paying stations to present promotional messages without appropriate disclosures, while their competitors observe the rules and present their content as properly acknowledged commercial advertisements. 3. The Bureau investigated a complaint that the Station broadcast announcements supporting a hydro-electronic energy project in New Hampshire without disclosing the identity of the company that sponsored the announcements. -

Federal Communications Commission DA 11-1546 Before the Federal
Federal Communications Commission DA 11-1546 Before the Federal Communications Commission Washington, D.C. 20554 In the Matter of ) ) Existing Shareholders of Cumulus ) BTC-20110330ALU, et al., Media, Inc. (Transferors) ) BTCH-20110331AIF, et al., and ) BTCH-20110331 AJF, et al., Existing Shareholders of Citadel ) BTCH-20110331AJN Broadcasting Corporation (Transferors) ) BTC-20110331AJO and ) BTCFT-20110331AKE, et al., New Shareholders of Cumulus Media, Inc. ) BTC-20110330ADE, et al., (Transferees) ) BTC-20110330ALJ, et al., ) BTCH-20110330ALM, et al., For Consent to Transfers of Control ) BTCH-20110330ALO, et al., ) BTCH-20110330AYC ) BTC-20110330AYD ) BTC-20110330AYF, et al., ) BTC-20110331AAA, et al., ) BTC-20110331AEV, ) BTC-20110331AEU ) BTC-20110331AEW ) BTCH-20110331AEX ) BTC-20110331AHZ, et al., ) BTCFT-20110510ADO, et al., ) Existing Shareholders of Cumulus ) BALH-20110331AID, et al., Media, Inc. ) BAL-20110331AJP, et al., (Assignors) ) BALH-20110331AJZ and ) BAL-20110331AKA Existing Shareholders of Citadel ) Broadcasting Corporation ) (Assignors) ) and ) Volt Radio, LLC, as Trustee ) (Assignee) ) ) For Consent to Assignment of Licenses ) MEMORANDUM OPINION AND ORDER Adopted: September 14, 2011 Released: September 14, 2011 By the Chief, Media Bureau: Federal Communications Commission DA 11-1546 I. INTRODUCTION 1. The Media Bureau (“Bureau”) has under consideration the captioned transfer and assignment applications (the “Applications”), as amended,1 in connection with a proposed transaction whereby a wholly-owned subsidiary of -

Licensing and Management System
Approved by OMB (Office of Management and Budget) 3060-0010 September 2019 (REFERENCE COPY - Not for submission) Amendment to a Commercial Broadcast Stations Biennial Ownership Report File Number: 0000101736 Submit Date: 2021-04-13 FRN: 0027643071 Purpose: Commercial Broadcast Stations Biennial Ownership Report Amendment Status: Received Status Date: 04/13/2021 Filing Status: Active Section I - General Information 1. Respondent FRN Entity Name 0027643071 SP Signal Manager, LLC Street City (and Country if non State ("NA" if non-U. Zip Address U.S. address) S. address) Code Phone Email Two Greenwich CT 06830 +1 (203) kmatthews@silverpointcapital. Greenwich 542-4274 com Plaza 2. Contact Name Organization Representative John S. Logan Cooley LLP Zip Street Address City (and Country if non U.S. address) State Code Phone Email 1299 Washington DC 20004 +1 (202) 776-2640 [email protected] Pennsylvania Avenue, NW Suite 700 Not Applicable 3. Application Filing Fee 4. Nature of (a) Provide the following information about the Respondent: Respondent Relationship to stations/permits Entity required to file a Form 323 because it holds an attributable interest in one or more Licensees Nature of Respondent Limited liability company (b) Provide the following information about this report: Purpose Biennial "As of" date 10/01/2019 When filing a biennial ownership report or validating and resubmitting a prior biennial ownership report, this date must be Oct. 1 of the year in which this report is filed. Reason for Amendment Addition of Stations Per FCC Request 5. Licensee(s) and Station(s) Respondent is filing this report to cover the following Licensee(s) and station(s): Licensee/Permittee Name FRN Radio License Holding SRC LLC 0023756331 Fac. -

Before the FEDERAL COMMUNICATIONS COMMISSION Washington, DC 20554
Before the FEDERAL COMMUNICATIONS COMMISSION Washington, DC 20554 In the Matter of ) ) Sponsorship Identification Rules and ) MB Docket No. 08-90 Embedded Advertising ) ) To: The Commission REPLY COMMENTS OF JOINT RADIO BROADCASTERS Beasley Broadcast Group, Inc., Citadel Broadcasting Corporation, Entercom Communications Corp., Greater Media, Inc. and Journal Broadcast Corporation, (collectively, the "Joint Radio Broadcasters"), by their attorneys, hereby reply to comments filed in response to the Notice of Inquiry and Notice of Proposed Rule Making ("NOIINPRM") in the above captioned proceeding. I Joint Radio Broadcasters collectively own and operate 441 radio stations located in markets of all sizes throughout the United States, as described in Appendix A. The NOIINPRM and the responsive comments have focused primarily on the placement, and potential additional regulation of, "embedded advertising" during television programming.2 In fact, the NOIINPRM requests comment on only one issue I Sponsorship Identification Rules and Embedded Advertising, Notice of Inquiry and Notice of Proposed Rule Making, 23 FCC Rcd 10682 (2008) ("NOIINPRM"). 2 The NOIINPRM defines embedded advertising as "situations where sponsored brands are included in entertainment programming," id. n.l, and attributes an "escalation" in such sponsorships "in part to recent technological changes that allow consumers to more readily bypass commercial content." !d. (lJ[I). Joint Radio Broadcasters note that these technological changes (particularly, the advent of digital video recorders) are centered on television, and that there is no evidence that "[t]he use of embedded advertising is escalating," id. (lJ[2), in the radio broadcast industry. 268130 exclusive to radio programming - - "radio hosts' personal, on-air endorsements of products or services that may have been provided at little or no cost to them,,,3 and generally considers radio programming together with television programming in inquiring about the need for modifications to sponsorship identification rules. -

Primary & Secondary Sources
Primary & Secondary Sources Brands & Products Agencies & Clients Media & Content Influencers & Licensees Organizations & Associations Government & Education Research & Data Multicultural Media Forecast 2019: Primary & Secondary Sources COPYRIGHT U.S. Multicultural Media Forecast 2019 Exclusive market research & strategic intelligence from PQ Media – Intelligent data for smarter business decisions In partnership with the Alliance for Inclusive and Multicultural Marketing at the Association of National Advertisers Co-authored at PQM by: Patrick Quinn – President & CEO Leo Kivijarv, PhD – EVP & Research Director Editorial Support at AIMM by: Bill Duggan – Group Executive Vice President, ANA Claudine Waite – Director, Content Marketing, Committees & Conferences, ANA Carlos Santiago – President & Chief Strategist, Santiago Solutions Group Except by express prior written permission from PQ Media LLC or the Association of National Advertisers, no part of this work may be copied or publicly distributed, displayed or disseminated by any means of publication or communication now known or developed hereafter, including in or by any: (i) directory or compilation or other printed publication; (ii) information storage or retrieval system; (iii) electronic device, including any analog or digital visual or audiovisual device or product. PQ Media and the Alliance for Inclusive and Multicultural Marketing at the Association of National Advertisers will protect and defend their copyright and all their other rights in this publication, including under the laws of copyright, misappropriation, trade secrets and unfair competition. All information and data contained in this report is obtained by PQ Media from sources that PQ Media believes to be accurate and reliable. However, errors and omissions in this report may result from human error and malfunctions in electronic conversion and transmission of textual and numeric data. -
Freq Call State Location U D N C Distance Bearing
AM BAND RADIO STATIONS COMPILED FROM FCC CDBS DATABASE AS OF FEB 6, 2012 POWER FREQ CALL STATE LOCATION UDNCDISTANCE BEARING NOTES 540 WASG AL DAPHNE 2500 18 1107 103 540 KRXA CA CARMEL VALLEY 10000 500 848 278 540 KVIP CA REDDING 2500 14 923 295 540 WFLF FL PINE HILLS 50000 46000 1523 102 540 WDAK GA COLUMBUS 4000 37 1241 94 540 KWMT IA FORT DODGE 5000 170 790 51 540 KMLB LA MONROE 5000 1000 838 101 540 WGOP MD POCOMOKE CITY 500 243 1694 75 540 WXYG MN SAUK RAPIDS 250 250 922 39 540 WETC NC WENDELL-ZEBULON 4000 500 1554 81 540 KNMX NM LAS VEGAS 5000 19 67 109 540 WLIE NY ISLIP 2500 219 1812 69 540 WWCS PA CANONSBURG 5000 500 1446 70 540 WYNN SC FLORENCE 250 165 1497 86 540 WKFN TN CLARKSVILLE 4000 54 1056 81 540 KDFT TX FERRIS 1000 248 602 110 540 KYAH UT DELTA 1000 13 415 306 540 WGTH VA RICHLANDS 1000 97 1360 79 540 WAUK WI JACKSON 400 400 1090 56 550 KTZN AK ANCHORAGE 3099 5000 2565 326 550 KFYI AZ PHOENIX 5000 1000 366 243 550 KUZZ CA BAKERSFIELD 5000 5000 709 270 550 KLLV CO BREEN 1799 132 312 550 KRAI CO CRAIG 5000 500 327 348 550 WAYR FL ORANGE PARK 5000 64 1471 98 550 WDUN GA GAINESVILLE 10000 2500 1273 88 550 KMVI HI WAILUKU 5000 3181 265 550 KFRM KS SALINA 5000 109 531 60 550 KTRS MO ST. LOUIS 5000 5000 907 73 550 KBOW MT BUTTE 5000 1000 767 336 550 WIOZ NC PINEHURST 1000 259 1504 84 550 WAME NC STATESVILLE 500 52 1420 82 550 KFYR ND BISMARCK 5000 5000 812 19 550 WGR NY BUFFALO 5000 5000 1533 63 550 WKRC OH CINCINNATI 5000 1000 1214 73 550 KOAC OR CORVALLIS 5000 5000 1071 309 550 WPAB PR PONCE 5000 5000 2712 106 550 WBZS RI -

DOC-309139A1.Pdf
FEDERAL COMMUNICATIONS COMMISSION FACT SHEET FY 2011 AM & FM RADIO STATION REGULATORY FEES Class of Fac. Id. Call Sign Licensee Name Service 84272 961115MD NORTHERN LIGHTS BROADCASTING, L.L.C. FM 87880 970724NE PAMPA BROADCASTERS INC FM 88463 970925MP KRJ COMPANY FM 89133 971022MC GEORGE S. FLINN, JR. FM 160321 DKACE INTERMOUNTAIN MEDIA, LLC AM 164193 DKAHA SOUTH TEXAS FM INVESTMENTS, LLC FM 164213 DKANM TANGO RADIO, LLC FM 166076 DKBWT MAGNOLIA RADIO CORPORATION FM 83886 DKCHC PACIFIC SPANISH NETWORK, INC. FM 33725 DKCLA M.R.S. VENTURES, INC. AM 161165 DKDAN INTERMOUNTAIN MEDIA, LLC AM 129546 DKEWA KM COMMUNICATIONS, INC. AM 170947 DKGQQ KEMP COMMUNICATIONS, INC. FM 165974 DKHES INNOVATED FACILITY SOLUTIONS, INC. FM 160322 DKIFO INTERMOUNTAIN MEDIA, LLC AM 170990 DKIXC NOALMARK BROADCASTING CORPORATION FM 164216 DKKUL-FM TANGO RADIO, LLC FM 164214 DKNOS TANGO RADIO, LLC FM 4236 DKOTN M.R.S. VENTURES, INC. AM 52619 DKPBQ-FM M.R.S. VENTURES, INC. FM 86857 DKRKD M.R.S. VENTURES, INC. FM 170967 DKRKP JER LICENSES, LLC FM 164191 DKTSX SOUTH TEXAS FM INVESTMENTS, LLC FM 70630 DKTTL W.H.A.M. FOR BETTER BROADCASTING, INC. FM 164197 DKXME SOUTH TEXAS FM INVESTMENTS, LLC FM 33726 DKZYP M.R.S. VENTURES, INC. FM 16551 DKZYQ M.R.S. VENTURES, INC. FM 160230 DNONE NORTH STAR BROADCASTING PARTNERS, LLC AM 160392 DNONE TONOPAH RADIO, LLC AM 48759 DWCMA PERIHELION GLOBAL, INC. AM 2297 DWCRW NATIONS RADIO, LLC AM 46952 DWCUG MULLIS COMMUNICATIONS, INC. AM 160767 DWDME WIRELESS FIDELITY OF NORTH AMERICA, INC. AM 71349 DWDOD WDOD OF CHATTANOOGA, INC. -

Broadcast Applications 3/29/2018
Federal Communications Commission 445 Twelfth Street SW PUBLIC NOTICE Washington, D.C. 20554 News media information 202 / 418-0500 Recorded listing of releases and texts 202 / 418-2222 REPORT NO. 29203 Broadcast Applications 3/29/2018 STATE FILE NUMBER E/P CALL LETTERS APPLICANT AND LOCATION N A T U R E O F A P P L I C A T I O N AM STATION APPLICATIONS FOR ASSIGNMENT OF LICENSE ACCEPTED FOR FILING MN BAL-20180326ABU KASM 33464 STARCOM, LLC Voluntary Assignment of License E 1150 KHZ MN , ALBANY From: STARCOM, LLC To: CRYSTAL MEDIA GROUP, LLC Form 314 FM STATION APPLICATIONS FOR ASSIGNMENT OF LICENSE ACCEPTED FOR FILING MN BALH-20180326ABV KDDG 33463 STARCOM, LLC Voluntary Assignment of License E 105.5 MHZ MN , ALBANY From: STARCOM, LLC To: CRYSTAL MEDIA GROUP, LLC Form 314 FM TRANSLATOR APPLICATIONS FOR ASSIGNMENT OF LICENSE ACCEPTED FOR FILING MN BALFT-20180326ABW K299BT 147518 STARCOM, LLC Voluntary Assignment of License E 107.7 MHZ MN , ALBANY From: STARCOM, LLC To: CRYSTAL MEDIA GROUP, LLC Form 314 FM AUXILIARY TRANSMITTING ANTENNA APPLICATIONS FOR AUXILIARY PERMIT ACCEPTED FOR FILING TX BXPH-20180326ABQ KGSR 23604 EMMIS AUSTIN RADIO CP for auxiliary purposes. BROADCASTING COMPANY, L.P. E 93.3 MHZ TX , CEDAR PARK Page 1 of 109 Federal Communications Commission 445 Twelfth Street SW PUBLIC NOTICE Washington, D.C. 20554 News media information 202 / 418-0500 Recorded listing of releases and texts 202 / 418-2222 REPORT NO. 29203 Broadcast Applications 3/29/2018 STATE FILE NUMBER E/P CALL LETTERS APPLICANT AND LOCATION N A T U R E O F A P P L I C A T I O N AM STATION APPLICATIONS FOR LICENSE TO COVER ACCEPTED FOR FILING MA BL-20180319BOB WNTN 48781 DELTA COMMUNICATIONS, LLC License to cover. -

Media Supporters (Updated September 21, 2013)
Media Supporters (Updated September 21, 2013) We greatly appreciate the generosity of the 9News, KUSA-TV, Denver which, was the first Colorado broadcast station to donate time for the airing of 9-1-1Colorado Foundation Public Service Announcements (“PSA”), and which also produced the PSA featuring Anchor/Reporter Gregg Moss. 9News airing of this PSA has resulted in the registration of thousands of Colorado residents for Emergency Notification Service. Public safety authorities use Emergency Notification Services to telephone, text and/or e-mail residents of areas threatened by wildfires, floods, tornados, chemical spills, police emergencies, etc., and provide warnings and instructions. You can view the 9News PSA on our Audio and Videos page. We also greatly appreciate the Colorado Broadcasters Association and its participating Member Stations for airing the Foundation’s public information announcements at a special rate. Our initial announcements also directed towards registration of Colorado residents for Emergency Notification Services. The participating stations include: KAJX-FM KBKL-FM KCJX-FM Aspen Grand Junction Carbondale KALC-FM KBLJ-AM KCME-FM Denver La Junta Manitou Springs KALQ-FM KBNO-AM KCNC-TV Alamosa Denver Denver KATC-FM KBPI-FM KCOL-AM Colorado Springs Denver Wellington KATR-FM KCCS-FM KCRT-AM Otis Starkville Trinidad KAVA-AM KCCY-AM KCRT-FM Pueblo Pueblo Trinidad KAVP-AM KCCY-FM KCSF-AM Colona Pueblo Colorado Springs KAYW-FM KCDO-TV KCSJ-AM Meeker Denver/Sterling Pueblo KBCO-FM KCEC-TV KCSU-FM Boulder Denver Fort Collins