NIH Public Access Author Manuscript Vaccine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Country – Peru
D AT E – 4/27/10 COUNTRY – PERU COMMODIT Y – Almonds & Pistachios BOTANICAL NAME – Prunus spp., Pistachia vera PRODUCT FORM – Nuts PHYTO FORM – Fed IMPORT PERMIT – Yes RESTRICTIONS – Prohibited SUMMARY INFO – Almonds with & without shells – Import Permit and Federal Phytosanitary Certificate Required. DO NOT CERTIFY UNLESS AN IMPORT PERMIT IS PRESENTED.. Pistachios – Import Permit and Federal Phytosanitary Certificate Required. DO NOT CERTIFY UNLESS AN IMPORT PERMIT IS PRESENTED. Fruit must enter through and authorized port of entry. Ports of entry for fruits and vegetables: 1) The International Airport of Lima-Callao 2) The Post Office at Arequipa, Chiclayo, Iquitos, Lima, Madre de Dios, Moquegua, Piura, Puno, Tacna, Trujillo, and Tumbes. 3) The airports at Arequipa, Chiclayo, Iquitos, Piura, Tacna, Trujillo, or Tumbes. 4) The ports are Aguas Verdes, Callao, Chicama, Eten, Ilo Iquitos, Matarani, Mollendo, Piata, Pimentel, Pisco, Puerto Maldonado, Puno, Salaverry, Tacna, Talara, Tumbes, and Yunguyo. SUBSIDIARY INFO – Phyto Certificates must be accurate and legible with no alterations or erasures and should always include the weight of the commodity in kilograms. Phyto inspections must be done within 30 days of shipment. COUNTRY – PERU D AT E – 4/21/10 COMMODIT Y – Apples, Grapes, Kiwi, Peaches, Pears BOTANICAL NAME – Malus domestica, Vitis vinifera, Actinidia chinensis, Prunus persica, Pyrus communis PRODUCT FORM – Fruit, Vegetables PHYTO FORM – Fed IMPORT PERMIT – Yes RESTRICTIONS – Prohibited SUMMARY INFO – (From CA) Import Permit and Federal Phytosanitary Certificate required. DO NOT CERTIFY UNLESS AN IMPORT PERMIT IS PRESENTED. Fruit must enter through and authorized port of entry. Ports of entry for fruits and vegetables: 1) The International Airport of Lima-Callao 2) The Post Office at Arequipa, Chiclayo, Iquitos, Lima, Madre de Dios, Moquegua, Piura, Puno, Tacna, Trujillo, and Tumbes. -

Peru | Flooding in Iquitos
Financing Experience in the Peruvian Amazon region Changing the paradigm to act faster FLOODING IN THE PERUVIAN AMAZON REGION River Amazon monitoring (Enapu) Every year parts of the Amazon forest flood from December to May. This floo- ding is caused by seasonal rains in the Peruvian Andes. Flow09_10 Flow10_11 Flooding can be extreme Flow11_12 Flow12_13 in some years due to chan- Flow13_14 Level ges in rainfall patterns that Flow14_15 are getting more extreme Flow15_16 and more frequent due to climate change. This cau- ses losses in infrastructure and livelihoods and affects people’s health. Oct 2016 Jan 2017 Apr 2017 Jul 2017 Date The largest recent floods came in 1999, 2012 and 2015. The highest record was 118.97m river level, in April 2012 at the Enapu station. Many communities were affected (see be- low); 26,000 hectares were flooded, as well as urban areas of the province Maynas. 2 2012 3,417 72,642 229,412 displaced affected victims families houses 2015 1,558 18,612 113,485 displaced affected victims families houses Source: INDECI THE CONCEPT OF FORECAST-BASED FINANCING Forecast-based financing (FbF) develops new procedures and methodologies to prepare vulnerable people facing a disaster in a more efficient and effective way. Using domestic and international hydro-meteorological forecast information, it sets danger levels and pre- defines early actions. These actions are activated when a forecast exceeds a danger level in the intervention area (for example, a river flow that has caused flooding in the past). Ac- tions can be implemented before the disaster occurs and resilience is strengthened, both at institutional and community level. -

LOCATION: Central Pacific Coast of South America
PERU *LOCATION: Central Pacific coast of South America. *GEOGRAPHY With 1,285,215 square km. Peru is the third-largest country in South America after Brazil and Argentina, ranking it amongst the world's 20 largest nations. Peru is divided into three regions: coast, mountains and jungle; also holds sway over the sea up to 200 miles from the coast and has territorial rights to an area of 60 million hectares in the Antarctic. It´s divided into 24 departments, being Lima the capital of the country. *POPULATION 29, 798, 000. *OFFICIAL LANGUAGE Spanish. Also English, French, spoken in most hotels and restaurants *TIME ZONE 5 hours behind the Greenwich Meridian Time (-5 GMT). *ELECTRICITY 220 V, 60Hz. It is recommendable to carry an adaptor for flat plugs. *CURRENCY The official currency in Peru is the Nuevo Sol (S/.), which is divided into 100 centimos. The currency includes coins for 5, 10, 20 and 50 centimos and 1, 2 and 5 sol coins. There are bills in the denomination of 10, 20, 50, 100 and 200 Nuevos Soles. *EXCHANGE RATE US$ to S/. 3.40 aprox € to S/. 3.80 *WEATHER IN LIMA °C: 22 – 18. °F 72 – 66. No precipitations. *PHONES AND COMMUNICATION Country area code: +51. Lima area code: 01. Peru features a far-reaching telephone network that provides services for national and international long-distance calls from private telephone lines and public cabins. There are also currently 1.3 million clients who have cellular phones, and satellite communications are currently being developed. *AIRPORTS Lima is 14 airports equipped to receive commercial flights and 10 ready for international flights: Lima, Arequipa, Chiclayo, Pisco, Pucallpa, Iquitos, Cusco, Trujillo, Tacna and Juliaca.17 airlines operating international flights and 7 airline companies, offering domestic flights *LOCAL TRANSPORTATION Use of public transportation, such as taxis or buses is common in Peru. -

MINISTERIO DE DESARROLLO E INCLUSIÓN SOCIAL a Través De FONDO DE COOPERACIÓN PARA EL DESARROLLO SOCIAL - FONCODES
MINISTERIO DE DESARROLLO E INCLUSIÓN SOCIAL a través de FONDO DE COOPERACIÓN PARA EL DESARROLLO SOCIAL - FONCODES Nombre del Programa: AYNI RAYMI Objetivo del programa: Rehabilitar y dar mantenimiento a la infraestructura de riego e infraestructura víal existente y generar empleo temporal en las áreas rurales pobres de la sierra y selva. Perfil de los participantes: Personas en situación de pobreza y pobreza extrema. Se trata de ejecución de proyectos en asociación con la población organizada y los gobiernos locales, comprenden obras de refacción y recuperación de canales de riego, Descripcion de las actividades: bocatomas, reservorios, caminos, puentes peatonales y trochas carrozables, entre otros. Se trata de un programa de intervención temporal que busca estimular las economías locales para mitigar los posibles impactos de la crisis económica internacional. FONCODES interviene en alrededor de 300 distritos en condición de pobreza y pobreza Acceso al Programa- Mecanismos extrema, y los proyectos se realizarán mediante la modalidad de Núcleo Ejecutor(*). Las de participacion obras, identificadas y priorizadas por las autoridades locales, se financiarán mediante las Oficinas Zonales. Apurímac, Ayacucho, Cajamarca, Amazonas, Cusco, Huancavelica, Junín, Huánuco, Ancash, Regiones donde se ejecuta el Lima, Piura, Puno, San Martín y La Libertad, Arequipa, Pasco, Lambayeque, Ica, Iquitos, programa: Moquegua, Ucayali, Madre de Dios, Tacna y Tumbes Teléfono en Lima: (01) 631 - 9000 Dirección: Paseo de la República 3101, San Isidro, Lima web: http://www.foncodes.gob.pe/ (*) Un nucleo Ejecutor (N.E.) es un órgano representativo de no menos de cien (100) personas de las comunidades campesinas y nativas, Asentamientos Humanos rurales y urbanos, así como rondas campesinas, comités de autodefensa, comités de gestión local, entre otros, que habiten en una determinada localidad rural o urbana en condición de pobreza. -

10-Day Amazon Expedition
10-day Amazon Expedition DAY 1: TRAVEL TO LIMA Travel from your hometown and arrive Lima, Peru. Overnight at the Costa del Sol Lima Airport hotel. DAY 2: LIMA TO IQUITOS/EXPLORAMA LODGE In the morning, fly to Iquitos, Peru—the heart of the Amazon! Meet your naturalist guides and board a covered boat for your 2-hour trip down the Amazon River and your first hike in the rainforest. DAY 3: EXPLORAMA LODGE Today, you’ll join the Yagua and learn about their traditional rainforest crafts and culture, including shooting blowguns and paddling dugout canoes. Try your hand at traditional dances and trading fish hooks and t-shirts for handmade Yagua crafts. Explore the Bushmaster Trail with your guide or visit the Amazon Library. Take a post-dinner night hike or boat ride in search of nocturnal wildlife. DAYS 4 and 5: EXPLORNAPO LODGE Day 4: At the mouth of the Napo River, search for pink and grey Amazon River dolphins. Be on the lookout for marmosets and tamarin monkeys who live near the dining room. Explore the ReNuPeru Ethnobotanical garden and learn about medicinal plants and traditional healing ceremonies with a local shaman. Get a huito fruit temporary tattoo of your favorite rainforest animal. Day 5: Spend the day in an Amazon village working side by side with community members on village improvement projects. Coordinated by the Peruvian non-profit CONAPAC, your day of service is also a day of celebration for the community and helps to support their Adopt-A-School program which delivers school supplies to over 50 remote villages in the Amazon! End your day with a game of soccer and celebratory humisha dance around a palm elaborately decorated with fruit and local crafts. -

Lima to Lima | 2021
2021 PERUVIAN RIVERS & RAINFOREST DISCOVERY 11 Days · Lima to Lima · Aria Amazon · November–May DAY PORT FEATURED EXCURSIONS HOTEL/SHIP Day 1 Lima Transfer from the Lima airport to the hotel Hotel Country Club Day 2 Lima Lima city tour [AM] Hotel Country Club Casa Aliaga [PM] Barranco tour [PM] Day 3 Lima Fly from Lima to Iquitos Iquitos Transfer from the Iquitos airport to the ship Amazon River Embark the ship Day 4 Amazon River Amazon to Yarapa River skiff ride [AM] Yarapa River Piranha Fishing & Lake Moringo [AM] Nauta Caño Wildlife & Stargazing in Nauta Caño [PM] Day 5 Marañon River Bird watching & jungle walk* [AM] Yanayacu-Pucate River Yanayuca Pucate River excursion [PM] Day 6 Nauta Nauta market visit [AM] Marañon River Birthplace of the Amazon River [PM] Day 7 Yanallpa River Yanallpa River skiff ride with jungle walk* [AM] Dorado River Dorado River skiff ride [PM] Day 8 Pacaya River Skiff tour on the Pacaya River [AM] Tapiche River Paiche fishing demonstration [PM] Ucayali River Ucayali River skiff tour [PM] Day 9 Yarapa or Clavero Lake Yarapa & Clavero Lake [PM] Amazon River Amazonian village visit [PM] Day 10 Nanay River Transfer from ship to the Iquitos airport via San Juan Disembark the ship Bellavista Nanay morning market [AM] Manatee rescue center [AM] San Juan craft market [AM] Iquitos Fly from Iquitos to Lima Lima Transfer from the Lima airport to the hotel Wyndham Costa del Sol Lima Airport Day 11 Lima Escort from the hotel to the Lima airport† Return Home PLEASE NOTE: Itinerary and hotels subject to change. -

Peru: an Andean Country with Significant Disaster and Emergency Management Challenges
Peru: An Andean Country with Significant Disaster and Emergency Management Challenges Heriberto Urby, Jr., J.D., Ph.D.1 David A. McEntire, Ph.D.2 Ekong J. Peters3 Introduction Peru is a poor, developing country in South America. Like so many other countries in the world, Peru has experienced its share of increasing and more intense disasters. Its emergency management system, while making progress in the past few years, remains weak in certain respects and in need of more proactive measures. In particular, the government and the emergency managers in this country need to continue to develop more effective approaches to implementation of the phases of emergency management (e.g., mitigation, preparedness, response, and recovery). This paper discusses Peru‘s disaster context; embarks on a short discussion of the vulnerability in this country; delineates the extreme past disasters experienced by her people; notes Peru‘s emergency management organization and laws, challenges and successes; and elaborates upon the lessons that have been learned as a result of studying Peru‘s emergency management system. The Peruvian Context Peru is located in South America and borders Chili to the south, Ecuador to the north, and Colombia, Brazil and Bolivia to the east. Its geography, climate, and people are diverse and somewhat unique. Peru‘s terrain includes relatively flat coastal areas, steep mountains in the central part of the country (rising to 5,000 meters or 16,400 feet in height in some cases), and rain forests in the east. The coastal areas are considered to be desert (particularly in the southern portion of the nation), while the central and eastern parts receive large amounts of rainfall. -

Concesión Del Primer Grupo De Aeropuertos De Provincia
INFORME DE DESEMPEÑO 2016 Concesión del Primer Grupo de Aeropuertos de Provincia Gerencia de Regulación y Estudios Económicos Contenido I. RESUMEN EJECUTIVO ........................................................................................ 4 II. ASPECTOS GENERALES DE LA ENTIDAD PRESTADORA ....................................... 6 II.1. Composición accionaria del Concesionario ........................................................... 6 II.2. Principales características de la Concesión ........................................................... 6 II.3. Principales activos ................................................................................................ 9 II.4. Área de influencia ................................................................................................. 9 II.5. Hechos importantes ........................................................................................... 11 III. OFERTA DE SERVICIOS PRESTADOS EN LA INFRAESTRUCTURA ......................... 12 IV. DEMANDA DE LOS SERVICIOS PRESTADOS ....................................................... 13 IV.1. Servicios aeroportuarios .................................................................................... 13 IV.2. Servicios no aeroportuarios ................................................................................ 21 V. REGULACIÓN TARIFARIA ................................................................................... 22 V.1. Tarifas reguladas ............................................................................................... -
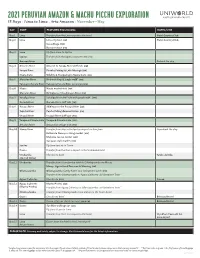
Lima to Lima | 2021
2021 PERUVIAN AMAZON & MACHU PICCHU EXPLORATION 15 Days · Lima to Lima · Aria Amazon · November–May DAY PORT FEATURED EXCURSIONS HOTEL/SHIP Day 1 Lima Transfer from the Lima airport to the hotel Hotel Country Club Day 2 Lima Lima city tour [AM] Hotel Country Club Casa Aliaga [PM] Barranco tour [PM] Day 3 Lima Fly from Lima to Iquitos Iquitos Transfer from the Iquitos airport to the ship Amazon River Embark the ship Day 4 Amazon River Amazon to Yarapa River skiff ride [AM] Yarapa River Piranha Fishing & Lake Moringo [AM] Nauta Caño Wildlife & Stargazing in Nauta Caño [PM] Day 5 Marañon River Bird watching & jungle walk* [AM] Yanayacu-Pucate River Yanayuca Pucate River excursion [PM] Day 6 Nauta Nauta market visit [AM] Marañon River Birthplace of the Amazon River [PM] Day 7 Yanallpa River Yanallpa River skiff ride with jungle walk* [AM] Dorado River Dorado River skiff ride [PM] Day 8 Pacaya River Skiff tour on the Pacaya River [AM] Tapiche River Paiche fishing demonstration [PM] Ucayali River Ucayali River skiff tour [PM] Day 9 Yarapa or Clavero Lake Yarapa & Clavero Lake [PM] Amazon River Amazonian village visit [PM] Day 10 Nanay River Transfer from ship to the Iquitos airport via San Juan Disembark the ship Bellavista Nanay morning market [AM] Manatee rescue center [AM] San Juan craft market [AM] Iquitos Fly from Iquitos to Cusco Cusco Transfer from the Cusco airport to the Urubamba hotel Urubamba Check in to hotel Tambo del Inka (Sacred Valley) Day 11 Urubamba Transfer from Urumbamba hotel to Ollantaytambo via Moray Moray–Agricultural -

Itinerary Or Similar, All Meals Picchu and Checkin at Our Hotel
WILD PERU ESCAPE: AMAZON & MACHU PICCHU Current route: Lima, Peru to Lima, Peru 9 Days Delfin II 28 Guests Expeditions in: Feb/Apr/Aug/Oct From $8,150 to $12,460 * Call us at 1.800.397.3348 or call your Travel Agent. In Australia, call 1300.361.012 • www.expeditions.com DAY 1: Lima, Peru padding Arrive in Lima, Peru’s thriving capital, and transfer 2022 Departure Dates: to the convenient Wyndham Costa del Sol Airport Hotel. You may also elect to arrive early for a Lima 12 Feb pre-voyage extension. Please call for details. 28 May 27 Aug 29 Oct DAY 2: Lima/Cusco/Sacred Valley padding Advance Payment: Arriving in Cusco, drive through the Sacred Valley to Chinchero, a typical Andean village where we $1,000 join National Geographic Grantee Nilda Callañaupa for lunch and a demonstration of Inca Cost Includes: weaving traditions. Continue to Ollantaytambo and All accommodations aboard ship or in board our late afternoon scenic train to Machu hotels per itinerary or similar, all meals Picchu and checkin at our hotel. We stay for two and nonalcoholic beverages aboard ship, nights at Inkaterra Machu Picchu Pueblo, with meals on land as indicated, shore Andean-style casitas set along the in a reserve excursions, sightseeing and entrance with 300 species of orchids. (B,L,D) fees, special access permits, transfers to and from group flights, use of kayaks and DAY 3: Machu Picchu skiffs, tips (except to ship’s crew), taxes padding Take the full day to explore Machu Picchu, the and service charges, services of a ship Inka royal retreat introduced to the world by Hiram medic and services of our expedition Bingham in the April 1913 National Geographic staff. -

Oficinas Y Puntos De Atención Para El Reconocimiento De Servicios Prepago
Oficinas y puntos de atención para el reconocimiento de servicios prepago Horario Lunes Horario Horario Nombre de Oficina Departamento Provincia Distrito Dirección Tipo a Viernes Sábado Domingo C.C. Open Plaza Angamos - Local A1, Av. CAC ANGAMOS LIMA LIMA SURQUILLO Angamos Cruce con Tomás Marsano CAC 11:00 - 21:00 11:00 - 21:00 11:00 - 19:00 CAC AREQUIPA AREQUIPA AREQUIPA YANAHUARA Av. El Ejército #701 CAC 09:00 - 20:00 09:00 - 19:00 - - - CAC AREQUIPA CERRO Centro Comercial Mall Center Arequipa – COLORADO AREQUIPA AREQUIPA CERRO COLORADO Av. Aviación Nro. 600 CAC 10:00 - 22:00 10:00 - 22:00 10:00 - 22:00 CAC AYACUCHO AYACUCHO HUAMANGA AYACUCHO Jr. Cuzco 220 CAC 10:00 - 19:00 10:00 - 14:00 - - - Av. Las Begonias #798, Esquina Con Rivera CAC BEGONIAS LIMA LIMA SAN ISIDRO Navarrete CAC 10:00 - 19:00 10:00 - 14:00 - - - C.C. Mall Plaza Bellavista - Av. Oscar R. Benavides N° 3866 - Locales B2003 – CAC BELLAVISTA LIMA CALLAO BELLAVISTA B2005 CAC 10:30 - 21:00 10:30 - 21:00 10:30 - 21:00 Centro Comercial La Rambla - Av. Brasil N° CAC BRASIL LIMA LIMA BREÑA 778 - Local R-01 2Do Piso CAC 10:00 - 21:00 10:00 - 21:00 10:00 - 21:00 C.C. El Quinde - Tiendas Lc301, Lc303 Y Le 120 - Jr. Sor Manuela Gil #151 Urb San CAC CAJAMARCA I CAJAMARCA CAJAMARCA CAJAMARCA Carlos CAC 10:00 - 22:00 10:00 - 22:00 10:00 - 22:00 C.C. Real Plaza Cajamarca Local Lc 205 Av. Evitamiento Esq. Prolong. Ayacucho Lote 1 CAC CAJAMARCA II CAJAMARCA CAJAMARCA CAJAMARCA - Barrio San Antonio CAC 10:00 - 21:00 10:00 - 21:00 11:00 - 21:00 Oficinas y puntos de atención para el reconocimiento de servicios prepago C.C. -

Casos De ZIKA - Perú 2017 Y 2017*
Casos de ZIKA - Perú 2017 y 2017* Mapa de ZIKA por distritos Perú 2017* Casos Autóctonos de enfermedad por virus Zika (Sospechosos + Confirmados) Perú 2016 Y 2017* 2016 2017* DIAGNOSTICOS DEPARTAMENTOS SOSPECHOSO CONFIRMADO TOTAL SOSPECHOSO CONFIRMADO TOTAL ZIKA ICA 1 3 4 3814 163 3977 LORETO 816 656 1472 959 108 1067 LIMA 0 1 1 194 46 240 TUMBES 7 4 11 70 36 106 LA LIBERTAD 0 0 0 31 4 35 PIURA 0 0 0 27 3 30 CAJAMARCA 0 17 17 8 3 11 UCAYALI 0 1 1 13 3 16 SAN MARTIN 0 2 2 9 2 11 MADRE DE DIOS 0 0 0 2 0 2 ZIKA ASINTOMATICO CAJAMARCA 0 12 12 0 0 0 LORETO 0 4 4 0 1 1 ZIKA ASINTOMATICO EN GESTANTES CAJAMARCA 0 31 31 0 0 0 LORETO 0 1 1 0 0 0 ZIKA EN GESTANTES LORETO 35 55 90 61 47 108 ICA 0 0 0 77 73 150 LIMA 0 0 0 3 2 5 CAJAMARCA 0 5 5 0 0 0 LAMBAYEQUE 0 0 0 1 0 1 TOTAL 859 792 1651 5269 491 5760 FUENTE : Centro Nacional de Epidemiologia, Prevención y Control de Enfermedades - MINSA (*) Hasta la SE 23 del 2017 Pirámide de casos de enfermedad por virus zika (EVZ) (Sospechoso + Confirmados) Perú – 2017* FUENTE : Centro Nacional de Epidemiologia, Prevención y Control de Enfermedades - MINSA (*) Hasta la SE 23 del 2017 Distribución de casos de enfermedad por virus zika (EVZ) Perú – 2016 – 2017* 1651 5760 FUENTE : Centro Nacional de Epidemiologia, Prevención y Control de Enfermedades - MINSA (*) Hasta la SE 23 del 2017 Casos de enfermedad por virus Zika confirmados (incluye sintomático y asintomático) Perú 2017 (*) DEPARTAMENTOS DISTRITOS casos % ICA PUEBLO NUEVO 120 24.4 CHINCHA ALTA 70 14.3 SUNAMPE 15 3.1 GROCIO PRADO 12 2.4 ICA 7 1.4 ALTO LARAN