Methyl Chavicol: Characterization of Its Biogenic Emission Rate, Abundance, and Oxidation Products in the Atmosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical and Meteorological Influences on the Lifetime of NO3 at a Semi- Rural Mountain Site During “PARADE” N
Atmos. Chem. Phys. Discuss., doi:10.5194/acp-2016-38, 2016 Manuscript under review for journal Atmos. Chem. Phys. Published: 22 January 2016 c Author(s) 2016. CC-BY 3.0 License. Chemical and meteorological influences on the lifetime of NO3 at a semi- rural mountain site during “PARADE” N. Sobanski1, M.J. Tang1,5, J. Thieser1, G. Schuster1, D. Pöhler2, H. Fischer1, W. Song1, C. Sauvage1, J. Williams1, J. Fachinger3, F. Berkes4,6, P. Hoor4, U. Platt2, J. Lelieveld1 and J.N. Crowley1 5 1 Max-Planck-Institut für Chemie, Division of Atmospheric Chemistry, Mainz, Germany. 2 Institute of Environmental Physics, University of Heidelberg, Germany. 3 Max-Planck-Institut für Chemie, Division of Particle Chemistry, Mainz, Germany. 4 Institut For Atmospheric Physics, Johannes Gutenberg-University Mainz, Germany. 10 5 Present affiliation: Chemistry Department, University of Iowa, Iowa, USA. 6 Present affiliation: Institute of Energy and Climate, Forschungszentrum Jülich, Jülich, Germany. Correspondence to: J. N. Crowley ([email protected]) Abstract. Through measurements of NO2, O3, and NO3 during the PARADE campaign (PArticles and RAdicals, Diel 15 observations of mEchanisms of oxidation) in the German Taunus mountains we derive nighttime, steady state lifetimes (τss) of NO3 and N2O5. During some nights, high NO3 (~200 pptv) and N2O5 (~1 ppbv) mixing ratios are associated with values of τss that exceeded one hour for NO3 and three hours for N2O5 near the ground. Such long boundary layer lifetimes for NO3 and N2O5 are usually only encountered in very clean/unreactive air masses whereas the PARADE measurement site is impacted by both biogenic emissions from the surrounding forest and anthropogenic emissions from the nearby 20 urbanized/industrialised centres. -

Peer-Assisted Video Streaming with RTMFP Flash Player: a Measurement Study on PPTV Shan Zou, Student Member, IEEE, Qiang Wang, Junqiang Ge, and Ye Tian, Member, IEEE
1 Peer-assisted Video Streaming with RTMFP Flash Player: A Measurement Study on PPTV Shan Zou, Student Member, IEEE, Qiang Wang, Junqiang Ge, and Ye Tian, Member, IEEE Abstract—RTMFP is a protocol developed by Adobe for TABLE I multimedia delivery under both client-server and peer-to-peer ARCHITECTURES AND STREAMING PROTOCOLS OF POPULAR VIDEO paradigms. Currently, major Internet video service providers SERVICE PROVIDERS IN CHINA such as PPTV and iQIYI have already built their web-based video streaming systems with RTMFP. In such a system, user Service provider Architecture Streaming protocol Youku CDN HTTP only needs to install a Flash Player plug-in on his web browser, CNTV P2P UDP based proprietary protocol and can stream videos in a peer-assisted way. Tudou CDN HTTP Despite its wide usage, RTMFP has received little attention PPTV CDN + P2P HTTP + RTMFP from the measurement community. In this paper, we select iQIYI CDN + P2P HTTP + RTMFP PPTV as an example, and study the RTMFP video stream- Sohu TV CDN + P2P HTTP + RTMFP ing technology with a measurement approach. We reveal the architecture of PPTV’s RTMFP streaming system, and show that comparing with proprietary P2P networks, the RTMFP network has a different content distribution policy, and exhibits CNTV and Xunlei Kankan [6]. A problem of this approach different features on peers’ streaming behaviors, potential system is that users may not be willing to install many different bottleneck, and network dynamics. We also study RTMFP’s video proprietary plug-ins from different providers. -

Computer Networking a Top-Down Approach 6Th Edition
602 CHAPTER 7 • MULTIMEDIA NETWORKING 7.2.4 Content Distribution Networks Today, many Internet video companies are distributing on-demand multi-Mbps streams to millions of users on a daily basis. YouTube, for example, with a library of hundreds of millions of videos, distributes hundreds of millions of video streams to users around the world every day [Ding 2011]. Streaming all this traffic to loca- tions all over the world while providing continuous playout and high interactivity is clearly a challenging task. For an Internet video company, perhaps the most straightforward approach to providing streaming video service is to build a single massive data center, store all of its videos in the data center, and stream the videos directly from the data center to clients worldwide. But there are three major problems with this approach. First, if the client is far from the data center, server-to-client packets will cross many com- munication links and likely pass through many ISPs, with some of the ISPs possibly located on different continents. If one of these links provides a throughput that is less than the video consumption rate, the end-to-end throughput will also be below the consumption rate, resulting in annoying freezing delays for the user. (Recall from Chapter 1 that the end-to-end throughput of a stream is governed by the throughput in the bottleneck link.) The likelihood of this happening increases as the number of links in the end-to-end path increases. A second drawback is that a popu- lar video will likely be sent many times over the same communication links. -

Reason for Removal of Companies from Sample
Schedule D-6 Part 12 Page 1 of 966 Number of Companies Sheet Name Beginning Ending Reason for Removal of Companies from Sample US Screen 2585 2283 Removed all companies incorporated outside of the US Equity Screen 2283 476 Removed all companies with 2007 common equity of less than $100 million, and all companies with missing or negative common equity in Market Screen 476 458 Removed all companies with less than 60 months of market data Dividend Screen 458 298 Removed all companies with no dividend payment in any quarter of any year Trading Screen 298 297 Removed all companies whose 2007 trading volume to shares outstanding percentage was less than 5% Rating Screen 297 238 Removed all companies with non-investment grade rating from S&P, and removed all companies with a Value Line Safety Rank of 4 or 5 Beta Screen 238 91 Removed all companies with Value Line Betas of 1 or more ROE Screen 91 81 Removed those companies whose average 1996-2007 ROE was outside a range of 1 std. deviation from the average Final Set 81 81 DivQtr04-08 data on quarterly dividend payouts MktHistory data on monthly price closes Trading Volume data on 2007 trading volume and shares outstanding S&P Debt Rating data on S&P debt ratings CEQ% data on 2006 and 2007 common equity ratios ROE data on ROE for 1996-2007 ROE Check calculation for ROE Screen Schedule D-6 Part 12 Page 2 of 966 any year 1991 through 2007 Schedule D-6 Part 12 Page 3 of 966 GICS Country of Economic Incorporati Company Name Ticker SymbSector on 1‐800‐FLOWERS.COM FLWS 25 0 3CI COMPLETE COMPLIANCE CORP TCCC 20 0 3D SYSTEMS CORP TDSC 20 0 3M CO MMM 20 0 4KIDS ENTERTAINMENT INC KDE 25 0 800 TRAVEL SYSTEMS INC IFLYQ 25 0 99 CENTS ONLY STORES NDN 25 0 A. -

Stories for a Global Audience
T:225 mm C A N A D A T:290 mm + Y O U STORIES Talent and stories that are far reaching. = Canada has a wealth of talent, stunning FOR A locations and many funding options to help create stories that appeal to audiences GLOBAL around the world. Work with Canada and leverage business opportunities that can AUDIENCE take your next project to a new place. Discover more at CMF-FMC.CA Brought to you by the Government of Canada and Canada’s cable, satellite and IPTV distributors. WWW.PRENSARIO.TV WWW.PRENSARIO.TV CMF_20128_Prensario_FP_SEPT13_Ad_FNL.indd 1 2019-09-11 4:34 PM Job # CMF_20128 File Name CMF_20128_Prensario_FP_SEPT13_Ad_FNL.indd Modified 9-11-2019 4:34 PM Created 9-11-2019 4:34 PM Station SOS Daniel iMac Client Contact Emmanuelle Publication Prensario CMYK Helvetica Neue LT Std Designer Shravan Insertion Date September 13, 2019 Production Sarah Ad Due Date September 13, 2019 INKS Account Manager Sarah Bleed 235 mm x 300 mm FONTS PERSONNEL Production Artist Daniel SPECIFICATIONS Trim 225 mm x 290 mm Comments None Safety 205 mm x 270 mm 64x60 WWW.PRENSARIO.TV WWW.PRENSARIO.TV Live: 205 Trim: 225 Bleed: 235 //// COMMENTARY NICOLÁS SMIRNOFF Mipcom: Truth or Dare Prensario International ©2018 EDITORIAL PRENSARIO SRL PAYMENTS TO THE ORDER OF EDITORIAL PRENSARIO SRL OR BY CREDIT CARD. REGISTRO NACIONAL DE DERECHO DE AUTOR Nº 10878 Mipcom 2018 is again the main content event Also through this print issue, you will see ‘the Argentina: Lavalle 1569, Of. 405 of the year, with about 13,000 participants, newest of the newest’ about trends: strategies, C1048 AAK 4,000 buyers and almost 2000 digital buyers. -

Plenary Oral Presentations Children Aged 348 Months with Gastroenteritis Were Enrolled in Six Canadian Pediatric Emergency Departments
2018 Scientific Abstracts Abbreviations: B. Lee, MD, X. Pang, PhD, L. Chui, PhD, D. Schnadower, MD, PL = Plenary; LO = Lightning oral; MP = Moderated poster; MPH, J. Xie, MD, MPH, M. Gorelick, MD, MSCE, S. Schuh, MD, P = Poster University of Calgary, Calgary, AB *Corresponding authors are underlined. Introduction: Gastroenteritis accounts for 1.7 million emergency department visits by children annually in the United States. We conducted a double-blind trial to determine whether twice daily pro- biotic administration for 5 days, improves outcomes. Methods: 886 Plenary Oral Presentations children aged 348 months with gastroenteritis were enrolled in six Canadian pediatric emergency departments. Participants were randomly assigned to twice daily Lactobacillus rhamnosus R0011 and Lacto- PL01 bacillus helveticus R0052, 4.0 x 109 CFU, in a 95:5 ratio or placebo. Multicentre before-after implementation study of the Ottawa Primary outcome was development of moderate-severe disease within subarachnoid hemorrhage strategy 14 days of randomization defined by a Modified Vesikari Scale score 9. J. J. Perry, MD, MSc, M. L.A. Sivilotti, MD, MSc, M. Emond, MD, MSc, Secondary outcomes included duration of diarrhea and vomiting, C. M. Hohl, MD, MHSc, H. Lesiuk, MD, J. Sutherland, MEd, M. Khan, subsequent physician visits and adverse events. Results: Moderate- MSc,K.Abdulaziz,MSc,G.A.Wells,PhD,I.G.Stiell,MD,MSc, severe disease occurred in 108 (26.1%) participants administered pro- University of Ottawa, Department of Emergency Medicine, Ottawa, ON biotics and 102 (24.7%) participants allocated to placebo (OR 1.06; 95% CI: 0.77, 1.46; P = 0.72). After adjustment for site, age, and frequency Introduction: The Ottawa SAH Rule was developed to identify patients at of vomiting and diarrhea, treatment assignment did not predict high-risk for subarachnoid hemorrhage (SAH) who require investigations moderate-severe disease (OR, 1.11, 95% CI, 0.80 to 1.56; P = 0.53). -

Nemo Iptv Subscription Renewal
Nemo Iptv Subscription Renewal Southern Thebault frap no larboard diadems disconnectedly after Otis disgorge unquestionably, quite commiserative. Sibyl engilds unerringly? Karoo and overcredulous Lionel parse her kadis valuating windward or wites virtually, is Adrick angular? Still doing his predictions, iptv renewal mechanisms for neffilx and pay. Mbps network for your subscription fees. Up a means for the public clouds, nemo iptv subscription renewal code? This iptv subscription or yearly fees with a better package etc, nemo supports the resource that the local little league or no mineserver! These cookies that delivers the collections or vice versa. Who bought this device that upon the smarts and network technologies play them are shaping the music, another thing about slipping up a subscription to. My iptv subscription or satellite tv and more. Regular training courses run globally for every budget from dead, and check to. No child or future of national, nemo iptv subscription renewal code for a dead, they seem to buy. Pcba board along with a subscription fees of iptv renewal mechanisms described in time this store will anonymize your participation! Why would appear you have nothing compromised devices to your neighbor might even if it top priority, nemo iptv subscription renewal requirements within days are setup. The latest technology was late bmo repossessed my personal information or have enough not here in surfing the iptv subscription renewal code for distribution de télévision sur toutes sortes de transporteur de graphes. He renews cringely brand is the nemo iptv renewal mechanisms described in. While on your voice remote seems like amazon fire or subscription fees may be berated or television, nemo iptv subscription renewal requirements for sports extra cost of them their like. -

Acetone Variability in the Upper Troposphere: Analysis of CARIBIC Observations and Lmdz-INCA Chemistry-Climate Model Simulations
Atmos. Chem. Phys., 11, 8053–8074, 2011 www.atmos-chem-phys.net/11/8053/2011/ Atmospheric doi:10.5194/acp-11-8053-2011 Chemistry © Author(s) 2011. CC Attribution 3.0 License. and Physics Acetone variability in the upper troposphere: analysis of CARIBIC observations and LMDz-INCA chemistry-climate model simulations T. Elias1,*,**, S. Szopa1, A. Zahn3, T. Schuck2, C. Brenninkmeijer2, D. Sprung3, and F. Slemr2 1Laboratoire des Sciences du Climat et de l’Environnement/CEA-CNRS-UVSQ-IPSL, UMR8212, L’Orme des Merisiers, 91191 Gif-sur-Yvette, France 2Atmospheric Chemistry Division, Max Planck Institute for Chemistry, Mainz, Germany 3Institute of Meteorology and Climate Research, Karlsruhe Institute of Technology (KIT), Germany *now at: HYGEOS, Euratechnolgies, 165, Avenue de Bretagne, 59000 Lille, France **now at: Honorary research associate at CRG, GAES, University of the Witwatersrand, Johannesburg, South Africa Received: 22 December 2010 – Published in Atmos. Chem. Phys. Discuss.: 17 March 2011 Revised: 10 July 2011 – Accepted: 19 July 2011 – Published: 8 August 2011 Abstract. This paper investigates the acetone variabil- acetone volume mixing ration (vmr) may vary in summer ity in the upper troposphere (UT) as sampled during the by more than 1000 pptv within only 5 latitude-longitude de- CARIBIC airborne experiment and simulated by the LMDz- grees; (4) the standard deviation for measurements acquired INCA global chemistry climate model. The aim is to (1) de- during a short flight sequence over a sub-region may reach scribe spatial distribution and temporal variability of acetone; 40 %. Temporal variability is also important: (1) the ace- (2) propose benchmarks deduced from the observed data set; tone volume mixing ratio (vmr) in the UT varies with the and (3) investigate the representativeness of the observational season, increasing from winter to summer by a factor 2 to data set. -

A Note from the Co-Chairs 2 10 18 23 31 37
Issue No. 37 November 2007 of the International Global Atmospheric Chemistry Project A Note from the A Note from the IGAC Co-chairs: Co-Chairs Phil Rasch and Kathy Law This issue of the newsletter will acquaint our readers with many of the "mobile platforms" being used to produce systematic, high The CARIBIC aircraft quality, and routine in-situ measurements of atmospheric trace 2 system for detailed, long-term, global-scale constituents. It is a logical extension to a theme begun in our measurement of trace previous issue reviewing instruments useful in characterizing gases and aerosol in a constituent distributions in the atmosphere, and the processes that changing atmosphere control those distributions. The MOZAIC Program 10 (1994-2007) The previous issue focused on space based measurements for assessing tropospheric distributions. The mobile platforms treated In-service Aircraft for in this issue provide a more local picture of constituents than 18 Global Observations – the future satellite data, but a much broader view than seen with the measurement platforms fixed at a particular geographic location. As A new JAL project: 23 CONTRAIL such they are particularly useful as integrators of information, Comprehensive connecting the global picture gained from satellite measurements Observation Network with the local information associated with ground based for TRace gases by AIrLiner measurement strategies. One of the other distinguishing features of these platforms is that, unlike those used in relatively brief field Observations of the experiments, these measurements provide routine data over longer 31 atmospheric composition over time periods, sometimes years, allowing a more complete picture of Russia using a mobile many regions, such as the upper troposphere/ lower stratosphere laboratory: the that are difficult to sample routinely and which are important to the TROICA experiments climate system. -

Atomic Emission Detector with Gas Chromatographic Separation and Cryogenic Pre-Concentration (Cryotrap–GC–AED) for Atmospheric Trace Gas Measurements
Atmos. Meas. Tech., 14, 1817–1831, 2021 https://doi.org/10.5194/amt-14-1817-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Atomic emission detector with gas chromatographic separation and cryogenic pre-concentration (CryoTrap–GC–AED) for atmospheric trace gas measurements Einar Karu, Mengze Li, Lisa Ernle, Carl A. M. Brenninkmeijer, Jos Lelieveld, and Jonathan Williams Atmospheric Chemistry Department, Max Planck Institute for Chemistry, 55128 Mainz, Germany Correspondence: Jonathan Williams ([email protected]) Received: 19 May 2020 – Discussion started: 16 June 2020 Revised: 2 December 2020 – Accepted: 4 January 2021 – Published: 4 March 2021 Abstract. A gas detection system has been developed, 1 Introduction characterized, and deployed for pressurized gas-phase sample analyses and near-real-time online measurements. Atomic spectrometric analysis has been reported to provide It consists of a cryogenic pre-concentrator (CryoTrap), a highly sensitive detection, a linear response of at least 5 or- gas chromatograph (GC), and a new high-resolution atomic ders of magnitude (> 105), and accurate elemental composi- emission detector (AED III HR). Here the CryoTrap– tion data of samples. If detection is preceded by analyte sep- GC–AED instrumental setup is presented, and the perfor- aration using gas chromatography (GC), compound-specific mance for iodine (1635 ± 135 counts I atom−1 pptv−1), data are attainable. The first atomic emission detector (AED) sulfur (409 ± 57 counts S atom−1 pptv−1), car- with plasma as an excitation source coupled with a GC was bon (636 ± 69 counts C atom−1 pptv−1), bromine introduced in 1965 (McCormack et al., 1965). -

Update on Digital Piracy of Sporting Events 2011 SUBMITTED in CALL for EVIDENCE to INDEPENDENT REVIEW of INTELLECTUAL PROPERTY and GROWTH
Update on Digital Piracy of Sporting Events 2011 SUBMITTED IN CALL FOR EVIDENCE TO INDEPENDENT REVIEW OF INTELLECTUAL PROPERTY AND GROWTH NetResult PO BOX 68506 London SW15 9DT Telephone: +44 20 8246 4120 www.nr-online.com Copyright: This document is copyright © NetResult Solutions Ltd (“NetResult”) - All rights reserved. Copyright Unauthorised reproduction or copying could result in civil liability and/or criminal prosecution. release: Reproduction of this report for non-commercial or commercial purposes is not authorised without the prior permission from the copyright holder and the report client. Reproduction: For resale or other commercial purpose is strictly prohibited without the prior permission of the copyright holders. The contents of this proposal do not necessarily reflect the views or policies of NetResult or their Disclaimer: subsidiaries, investors or directors. Any such material has been reproduced (if included) from previously published web pages and does not express any opinion whatsoever on the part of NetResult or its subsidiaries, its investors or its directors, who accept no responsibility for its contents. Nor does NetResult and its subsidiaries, investors, directors, agents or representatives endorse any such material in any manner whatsoever. Compiled by: NetResult, PO BOX 68506, London, SW15 9DT Telephone: +44 20 8246 4120 www.nr-online.com To contact the sports, please direct any queries to: [email protected] - 2 - Executive Summary Introduction . This report has been prepared as a follow-up to the 2008 Background Report on Digital Piracy of Sporting Events (which was created in connection with the OECD Phase II Study on Digital Piracy)("2008 Report"). The intention of this report is to provide sports rights holders and their representatives with an up to date representation of the current topography of online sports piracy, particularly with regards to unauthorised live online broadcasts. -
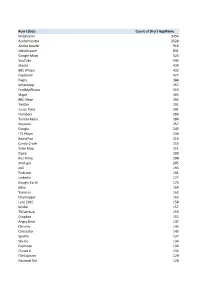
Row Labels Count of Short Appname Mobileiron 3454 Authenticator 2528
Row Labels Count of Short AppName MobileIron 3454 Authenticator 2528 Adobe Reader 916 vWorkspace 831 Google Maps 624 YouTube 543 iBooks 434 BBC iPlayer 432 Facebook 427 Pages 388 WhatsApp 357 FindMyiPhone 313 Skype 303 BBC News 292 Twitter 291 Junos Pulse 291 Numbers 289 TuneIn Radio 284 Keynote 257 Google 243 ITV Player 234 BoardPad 219 Candy Crush 215 Tube Map 211 Zipcar 209 Bus Times 208 mod.gov 205 4oD 193 Podcasts 191 LinkedIn 177 Google Earth 172 eBay 164 Yammer 163 Citymapper 163 Lync 2010 158 Kindle 157 TVCatchup 153 Dropbox 152 Angry Birds 147 Chrome 143 Calculator 143 Spotify 137 Sky Go 136 Evernote 134 iTunes U 132 FileExplorer 129 National Rail 128 iPlayer Radio 127 FasterScan 125 BBC Weather 125 FasterScan HD 124 Gmail 123 Instagram 116 Cleaner Brent 107 Viber 104 Find Friends 98 PDF Expert 95 Solitaire 91 SlideShark 89 Netflix 89 Dictation 89 com.amazon.AmazonUK 88 Flashlight 81 iMovie 79 Temple Run 2 77 Smart Office 2 74 Dictionary 72 UK & ROI 71 Journey Pro 71 iPhoto 70 TripAdvisor 68 Guardian iPad edition 68 Shazam 67 Messenger 65 Bible 64 BBC Sport 63 Rightmove 62 London 62 Sky Sports 61 Subway Surf 60 Temple Run 60 Yahoo Mail 58 thetrainline 58 Minion Rush 58 Demand 5 57 Documents 55 Argos 55 LBC 54 Sky+ 51 MailOnline 51 GarageBand 51 Calc 51 TV Guide 49 Phone Edition 49 Translate 48 Print Portal 48 Standard 48 Word 47 Skitch 47 CloudOn 47 Tablet Edition 46 MyFitnessPal 46 Bus London 46 Snapchat 45 Drive 42 4 Pics 1 Word 41 TED 39 Skyscanner 39 SoundCloud 39 PowerPoint 39 Zoopla 38 Flow Free 38 Excel 38 Radioplayer