What Are Alcohol Hand Sanitizers? Is It Safe to Handle Food After Using An
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hand Hygiene Solutions
PRODUCT OVERVIEWS Hand Hygiene Solutions Healing Hands Need Help, Too. Almost 80% of infectious Hand hygiene compliance Product selection matters diseases are transmitted rates are often very low Products containing aloe vera, via touch Outpatient clinics: Studies vitamin E and other emollients One of the most common have shown that hand hygiene can help to soothe and protect modes of transmission is frequency amongst clinic staff hands and even promote hand via the hands of healthcare ranges from 11-50%.2, 3 hygiene compliance.4 professionals.1 Designed for the demanding needs of healthcare professionals, Clorox Healthcare® provides a wide array of hand hygiene solutions formulated for frequent hand hygiene in healthcare settings. References 1. Tierno, P. The Secret Life of Germs. New York, NY, USA: Atria Books, 2001. 2. Kukanich KS, Kaur R, Freeman LC, Powell DA. Evaluation of a hand hygiene campaign in outpatient health care clinics. Am J Nurs. 2013 Mar;113(3):36-42. 3. Thompson D, Bowdey L, Brett M, Cheek, Using medical student observers of infection prevention, hand hygiene, and injection safety in outpatient settings: A cross-sectional survey. J Am J Infect. Control 2016; 44(4):374–380. 4. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. 2009. https://www.ncbi.nlm.nih.gov/books/NBK144051/ (Accessed 22 February 2017) 5. CDC. “Handwashing: Clean Hands Save Lives-Show Me the Science-Why Wash Your Hands?” https://www.cdc.gov/handwashing/why-handwashing.html (Accessed 15 March 2017). Hand hygiene is one of the most effective ways to stop the spread of germs and keep patients, staff and visitors safe.5 Our portfolio of hand hygiene solutions can be used to clean and soothe hands throughout your facility-from reception areas, to patient care areas to break rooms and offices. -

Ethanol Rins and Hand Sanitizer
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY NATIONAL VEHICLE AND FUEL EMISSIONS LABORATORY 2565 PLYMOUTH ROAD ANN ARBOR, MICHIGAN 48105-2498 OFFICE OF AIR AND RADIATION May 11, 2020 Re: Ethanol RINs and Hand Sanitizer Production for EP3 Pathway Ethanol Facilities during the COVID-19 Public Health Emergency Dear Ethanol Producers: EPA administers the Renewable Fuel Standard (RFS) program, including determining what volumes of fuel ethanol qualify for tradable credits (RINs) under the program. In 2014, as part of the RFS Program in 40 C.F.R. Part 80, we created the Efficient Producer Petition Process (EP3) as a streamlined process for corn starch and grain sorghum ethanol producers to demonstrate that their fuel satisfies the lifecycle greenhouse gas (GHG) reduction requirements to qualify for RINs. Consistent with 40 C.F.R. § 80.1416, over 80 dry mill ethanol plants in the United States have been approved for EP3 pathways. Given the current 2019 coronavirus disease (COVID-19), we understand that some consumers and health care professionals are currently experiencing difficulties accessing alcohol-based hand sanitizers. To enhance the availability of hand sanitizer products, the Food and Drug Administration (FDA) has issued a number of temporary policies, including temporary guidance for producers of alcohol for hand sanitizer.1 Based on input from industry, we understand fuel ethanol facilities can quickly and significantly enhance the availability of alcohol suitable for hand sanitizer production. Ethanol production through an EP3 pathway is limited to 200-proof ethanol, whereas alcohol suitable for use as an ingredient in hand sanitizer (hand sanitizer alcohol) is commonly produced as 190-proof ethanol. -

Hand Hygiene: Clean Hands for Healthcare Personnel
Core Concepts for Hand Hygiene: Clean Hands for Healthcare Personnel 1 Presenter Russ Olmsted, MPH, CIC Director, Infection Prevention & Control Trinity Health, Livonia, MI Contributions by Heather M. Gilmartin, NP, PhD, CIC Denver VA Medical Center University of Colorado Laraine Washer, MD University of Michigan Health System 2 Learning Objectives • Outline the importance of effective hand hygiene for protection of healthcare personnel and patients • Describe proper hand hygiene techniques, including when various techniques should be used 3 Why is Hand Hygiene Important? • The microbes that cause healthcare-associated infections (HAIs) can be transmitted on the hands of healthcare personnel • Hand hygiene is one of the MOST important ways to prevent the spread of infection 1 out of every 25 patients has • Too often healthcare personnel do a healthcare-associated not clean their hands infection – In fact, missed opportunities for hand hygiene can be as high as 50% (Chassin MR, Jt Comm J Qual Patient Saf, 2015; Yanke E, Am J Infect Control, 2015; Magill SS, N Engl J Med, 2014) 4 Environmental Surfaces Can Look Clean but… • Bacteria can survive for days on patient care equipment and other surfaces like bed rails, IV pumps, etc. • It is important to use hand hygiene after touching these surfaces and at exit, even if you only touched environmental surfaces Boyce JM, Am J Infect Control, 2002; WHO Guidelines on Hand Hygiene in Health Care, WHO, 2009 5 Hands Make Multidrug-Resistant Organisms (MDROs) and Other Microbes Mobile (Image from CDC, Vital Signs: MMWR, 2016) 6 When Should You Clean Your Hands? 1. Before touching a patient 2. -

FDA Warns That Vapors from Alcohol-Based Hand Sanitizers Can Have Side Effects Apply Hand Sanitizer in a Well-Ventilated Area
FDA warns that vapors from alcohol-based hand sanitizers can have side effects Apply hand sanitizer in a well-ventilated area 6-16-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that symptoms such as headache, nausea, and dizziness can occur after applying alcohol-based hand sanitizers to the skin. These symptoms are likely to have occurred because of vapors from the hand sanitizer, potentially from exposure in enclosed spaces or places with poor air circulation. We have received increasing reports of these side effects since the start of the COVID-19 pandemic. Most people experienced minor or minimal effects; however, some cases required treatment by a health care professional. What is FDA doing? We are continuing to monitor reports of adverse events that occur with hand sanitizers. At this time, we are not making any changes to the Drug Facts label for hand sanitizers but will inform the public if additional information becomes available. What are hand sanitizers and how can they help me? Hand sanitizers are over-the-counter (OTC) drug products that can help consumers reduce bacteria on their hands. However, the best way to prevent the spread of infections and decrease the risk of getting sick is by washing your hands with plain soap and water, advises the Centers for Disease Control and Prevention (CDC). Washing hands often with soap and water for at least 20 seconds is essential, especially after going to the restroom; before eating; and after coughing, sneezing, or blowing one’s nose. -

Safety Data Sheet Hand Sanitizer Disinfectant Sds
DISINFECTANT SDS STARTS ON University of Nebraska–Lincoln SAFETY DATA SHEET PAGE 9 - SCROLL DOWN HAND SANITIZER SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : HAND SANITIZER Other means of identification : not applicable Recommended use : Hand Sanitizer Restrictions on use : Reserved for industrial and professional use. Product dilution information : Product is ready to use. Company : University of Nebraska - Lincoln Nebraska Innovation Campus Lincoln, NE 1-402-472-2142 Emergency telephone : 1-800-424-9300 (US) Issuing date : 04/7/2020 SECTION 2. HAZARDS IDENTIFICATION GHS Classification Flammable liquids : Category 3 Eye irritation : Category 2A GHS Label element Hazard pictograms : Signal Word : Warning Hazard Statements : Flammable liquid and vapor. Causes serious eye irritation. Precautionary Statements : Prevention: Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Keep container tightly closed. Response: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/ attention. In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction. Storage: Store in a well-ventilated place. Keep cool. Disposal: Dispose of contents/ container to an approved waste disposal plant. Other hazards : None known. SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS Pure substance/mixture : Mixture 1 / 8 SAFETY DATA SHEET HAND SANITIZER (Bitrex added to denature) Chemical Name CAS-No. Concentration (%) ethanol 64-17-5 80% hydrogen peroxide 7722-84-1 < 1% glycerin 56-81-5 < 2% water 7732-18-5 17-18% SECTION 4. FIRST AID MEASURES In case of eye or skin contact : Rinse with water. -

Assessment of Antibacterial Efficacy of Lugol Iodine Compared With
2019SCIENCELINE Journal of Life Science and Biomedicine J Life Sci Biomed, 9 (5): 130-137, 2019 License: CC BY 4.0 ISSN 2251-9939 Assessment of antibacterial efficacy of Lugol's iodine compared with commercial hand sanitizers of Bangladesh Md. Nafiur Rahman1, Mohammad Abdullah-Al-Shoeb1, Saaimatul Huq2 and Muhammad Abul Kalam Azad1 1Department of Biochemistry and Molecular Biology, Shahjalal University of Science and Technology, Sylhet-3114, Bangladesh 2Molecular Biotechnology division, National Institute of Biotechnology, Savar, Dhaka-1349, Bangladesh Corresponding author's Email: [email protected] ABSTRACT Introduction. Hand disinfection is an essential step to prevent infection, reduce morbidity Research Article 's and minimize health care costs in a community. Aim. In this study, the Lugol iodine (2%) PII: S225199391900021-9 solution was evaluated to use as an emergency hand sanitizer and compared with the three commercially available hand sanitizers (Hexisol, Sepnil and Handirub) of Bangladesh. Methods. These hand sanitizers were examined and analyzed by susceptibility test, Rec. 13 July 2019 minimum bactericidal concentration test and efficacy determination test. The agar Rev. 20 September 2019 diffusion test was used to assess the efficacy of the products against pathogenic Escherichia Pub. 25 September 2019 coli, Shigella flexneri, Staphylococcus aureus, Salmonella typhi and Streptococcus pneumoniae. Results. Handirub has inhibited all the test organisms with highest zones of inhibition ranging between 24.38 mm and 28.63 mm while Hexisol zone of inhibition was ranging from 13.3 mm to 15 mm. Unfortunately, Sepnil was inactive against Salmonella typhi, with very poor performance against other test organisms. All the three commercial hand sanitizers Keywords were only bacteriostatic at 100% concentration, while both 2% and 1% iodine were 100% Hand sanitizer, bactericidal. -

Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency Immediately in Effect Guidance for Industry
Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency Immediately in Effect Guidance for Industry FDA is issuing this guidance for immediate implementation in accordance with 21 CFR 10.115(g)(2). Comments may be submitted at any time for Agency consideration. Submit written comments to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. Submit electronic comments to https://www.regulations.gov. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this document, contact FDA’s human drug compounding team (CDER) at [email protected]. March 2020 Updated February 10, 2021 Compounding Contains Nonbinding Recommendations Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency Immediately in Effect Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353 Email: [email protected] https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research -

Respiratory Syncytial Virus (RSV) Fact Sheet
Fact Sheet Respiratory Syncytial Virus (RSV) What is respiratory syncytial virus (RSV)? RSV is a major cause of respiratory illness in people, most common in infants and young children, but may infect adults. Most people experience mild illness and recover in 1-2 weeks, but some people can become very ill and require hospital care. People at greatest risk for severe illness include children under the age of 2, older adults especially those 65 years old or older, and people (of any age) who have heart or lung conditions or a weakened immune system. What are the symptoms of RSV? Begins with fever, runny nose, cough and sometimes wheezing Lethargy, irritability and poor feeding Chronic lung conditions and asthma may be aggravated by RSV Infants and children with heart or respiratory problems are at increased risk of pneumonia and other complications from RSV *If your baby is having difficulty breathing or feeding, seek medical attention. What is the treatment for RSV? There is no specific treatment for RSV. Health care providers may prescribe over-the-counter medication such as Tylenol to manage symptoms such as fever and pain. It is important for people with RSV infection to drink enough fluids to prevent dehydration. Those at risk for severe disease may need to be hospitalized if they are dehydrated or have trouble breathing. How is RSV spread? Through close contact, when an infected person person sneezes or coughs and the virus gets into your body through your eyes, nose or mouth By touching infected surfaces or objects that the virus has landed on, and then touching your face without washing your hands The virus can survive on surfaces/objects (phones, toys, doorknobs) for many hours For how long is an infected person able to spread RSV? Infected individuals spread RSV for as long as they are ill (usually 3-8 days). -

Clean Hands Count for Healthcare Providers Factsheet
FOR HEALTHCARE PROVIDERS KNOW THE TRUTH TO PROTECT YOURSELF AND PROTECT YOUR PATIENTS TRUTH: TRUTH: Alcohol-based hand sanitizer is Alcohol-based hand sanitizer more effective and less drying does not kill C. difficile, but it than using soap and water. is still the overall recommended method for hand hygiene THE NITTY GRITTY: Compared to soap and water, alcohol- practice. based hand sanitizers are better at reducing bacterial counts on hands and are effective THE NITTY GRITTY: against multidrug-resistant organisms (e.g., Always use gloves when caring for patients MRSA). Additionally, alcohol-based hand with C. difficile. In addition, when there is sanitizers cause less skin irritation than an outbreak of C. difficile in your facility, frequent use of soap and water. wash your hands with soap and water after removing your gloves. MORE LESS TRUTH: EFFECTIVE DRYING Some healthcare providers miss certain areas when cleaning their hands. THE NITTY GRITTY: Using alcohol-based hand sanitizer becomes a BAD GERMS GOOD GERMS habit and sometimes healthcare providers miss certain areas: TRUTH: FINGERTIPS Using alcohol-based hand sanitizer does NOT cause antibiotic resistance. THE NITTY GRITTY: Alcohol-based hand sanitizers kill germs quickly and in a different way than antibiotics. There is no chance for the germs to adapt or develop THUMBS resistance. BETWEEN FINGERS Clean Hands Count 100% of the Time PROTECT YOURSELF AND PROTECT YOUR PATIENTS FROM POTENTIALLY DEADLY GERMS TRUTH: The amount of product you use matters. THE NITTY GRITTY : Use enough alcohol-based hand sanitizer to cover all surfaces of your hands. Rub your hands together until they are dry. -

Clostridioides Difficile
PATIENT & CAREGIVER EDUCATION Clostridioides Difficile This information explains infection with Clostridioides difficile (C. diff), including how it’s spread and how it’s treated. What is Clostridioides difficile? Clostridioides difficile, or C. diff, is a germ that causes an infection in your colon. The infection gives you diarrhea (loose or watery bowel movements) and colitis. Colitis is an inflammation (swelling and redness) of your colon. How does C. diff spread? C. diff is spread by direct contact with an infected person’s bowel movement (stool). It can also be spread by contact with equipment or surfaces that may have the germ on them. Casual contact, such as touching or hugging, doesn’t spread C. diff. Who is at risk for a C. diff infection? C. diff infections occur more often in people who: Are older Have weakened immune systems Have chronic illnesses, such as cancer and diabetes Have been treated with antibiotics in the past Have had abdominal surgery Have had repeated or long stays in the hospital Have low stomach acid or have taken antacids (medication to reduce stomach Clostridioides Difficile 1/4 acid) What are the symptoms of a C. diff infection? Diarrhea is the main symptom of mild cases of C. diff infections. Symptoms of more severe cases include abdominal (belly) cramps and diarrhea with blood and mucus. How is a C. diff infection treated? C. diff infection is treated with antibiotics. The usual treatment is metronidazole (Flagyl® ), which is taken for 7 to 14 days. What isolation precautions are taken in the hospital if I have a C. -
Clostridium Difficile Infection
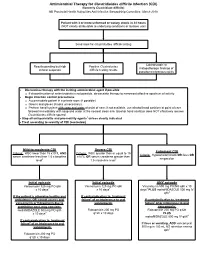
Antimicrobial Therapy for Clostridioides difficile Infection (CDI) (formerly Clostridium difficile) NB Provincial Health Authorities Anti-Infective Stewardship Committee, March 2019 Patient with 3 or more unformed or watery stools in 24 hours (NOT clearly attributable to underlying conditions or laxative use) Send stool for Clostridioides difficile testing Colonoscopic or Results pending but high Positive Clostridioides histopathologic findings of clinical suspicion difficile testing results pseudomembranous colitis Discontinue therapy with the inciting antimicrobial agent if possible o If discontinuation of antimicrobials is not possible, de-escalate therapy to narrowest effective spectrum of activity Begin infection control precautions o Accommodate patient in a private room (if possible) o Gowns and gloves (masks unnecessary) o Perform hand hygiene with soap and water at point of care; if not available, use alcohol hand sanitizer at point of care followed immediately with soap and water at the nearest clean sink (alcohol hand sanitizer does NOT effectively remove Clostridioides difficile spores) Stop all anti-peristaltic and pro-motility agents1 unless clearly indicated Treat according to severity of CDI (see below) Mild-to-moderate CDI Severe CDI Fulminant CDI Criteria: WBC lower than 15 x109/L AND Criteria: WBC greater than or equal to 15 Criteria: Hypotension/shock OR ileus OR serum creatinine less than 1.5 x baseline x109/L OR serum creatinine greater than megacolon level2 1.5 x baseline level2 Initial episode Initial episode -

Hand Washing Vs Hand Sanitizer: Which Is Better at Fighting COVID-19?
Media Contact Kara Spak [email protected] 312-593-0596 (cell) Hand washing vs Hand Sanitizer: Which is better at fighting COVID-19? Attribute to: Melinda Ring, MD, executive director at the Osher Center for Integrative Medicine at Northwestern Medicine We’ve heard the single most important way to prevent COVID-19 infection is to wash our hands with soap and water, and to use alcohol-based hand sanitizer. Dr. Melinda Ring, executive director of the Osher Center for Integrative Medicine at Northwestern Medicine, answers frequently asked questions about keeping hands clean. Question: What is better, washing hands with soap and water or hand sanitizer? Are they equally good at keeping our hands clean? Melinda Ring, MD According to the Centers for Disease Control (CDC), washing hands with Integrative Medicine soap and water is preferable to sanitizing gel. The gel may not be as Northwestern Memorial effective as soap in terms of eliminating all types of germs, including some Hospital viruses. Alcohol based gels and foams where the alcohol concentration is at least 60% can be effective- as long as someone uses it properly. Studies show that often people don't apply enough gel, or wipe it off before it dries. The time required for complete drying ideally is 30 seconds or less, but in some cases it can take over 100 seconds to dry (doesn’t sound like a lot, but people tend to reach for a towel or wipe their hands on their pants instead of waiting). Question: Any tips for making sure you use hand sanitizer properly? While there are detailed protocols available for how to ensure you cover the whole hand surface, for most people just knowing that they should apply the product to the palm of one hand, using the label-recommended amount, and rub the product all over the surfaces of your hands until your hands are dry should be adequate.