The Aging Enigma Scientists Probe the Genetic Basis of Longevity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
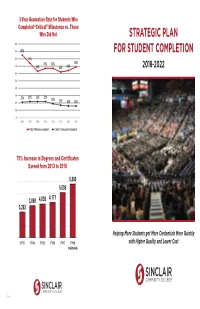
Strategic Plan for Student Completion
3-Year Graduation Rate for Students Who Completed “Critical” Milestones vs. Those Who Did Not STRATEGIC PLAN 100% 90% 85% FOR STUDENT COMPLETION 80% 74% 67% 67% 69% 70% 63% 62% 64% 2018-2022 60% 50% 40% 30% 21% 22% 22% 22% 19% 20% 17% 16% 16% 10% 0% AY06 AY07 AY08 AY09 AY10 AY11 AY12 AY13 ΗƌŝƚŝĐĂůΗDŝůĞƐƚŽŶĞƐ Completed ΗƌŝƚŝĐĂůΗDŝůĞƐƚŽŶĞƐNot Completed 76% Increase in Degrees and Certifi cates Earned from 2013 to 2018 Helping More Students get More Credentials More Quickly with Higher Quality and Lower Cost 285/16/2018 | More Students | More Credentials | More Quickly | Higher Quality & Lower Cost More Students | More Credentials | More Quickly | Higher Quality & Lower Cost | 1 Students Completing Nine Credit Hours in the First Year in their Declared Major 60% INTRODUCTION 50% 46% 42% 40% Sinclair has been working on student success While hundreds of faculty and staff have 33% since its inception 130 years ago. While the focus contributed to the creation of this focused plan, 31% 30% originally was on providing access to students the following are more directly responsible for who may not otherwise be able to earn a valuable creating this document: 20% 19% 20% 19% credential, the college sought to close the skills gap 20% between available talent and the needs of business • Carol Bonner 15% 16% 12% and indus- try. David Sinclair's motto of "Find • Mike Brigner Goal for 2022: 10% the Need and Endeavor to Meet it" still guides the • Dr. Kathleen Cleary 60% college's planning today. • Dr. Dave Collins We have learned that time is the enemy for our 0% • Laura Hinkebein AY06 AY07 AY08 AY09 AY10 AY11 AY12 AY13 AY14 AY15 AY16 students: if they take too long to graduate, life • Dawayne Kirkman will inevitably get in the way. -

Christopher Upton Phd Thesis
?@A374? 7; ?2<@@7?6 81@7; 2IQJRSOPIFQ 1$ APSON 1 @IFRJR ?TCMJSSFE GOQ SIF 3FHQFF OG =I3 BS SIF ANJUFQRJSX OG ?S$ 1NEQFVR '.-+ 5TLL MFSBEBSB GOQ SIJR JSFM JR BUBJLBCLF JN >FRFBQDI0?S1NEQFVR/5TLL@FWS BS/ ISSP/%%QFRFBQDI#QFPORJSOQX$RS#BNEQFVR$BD$TK% =LFBRF TRF SIJR JEFNSJGJFQ SO DJSF OQ LJNK SO SIJR JSFM/ ISSP/%%IEL$IBNELF$NFS%'&&()%(,)* @IJR JSFM JR PQOSFDSFE CX OQJHJNBL DOPXQJHIS STUDIES IN SCOTTISH LATIN by Christopher A. Upton Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at the University of St. Andrews October 1984 ýýFCA ýý£ s'i ý`q. q DRE N.6 - Parentibus meis conjugique meae. Iý Christopher Allan Upton hereby certify that this thesis which is approximately 100,000 words in length has been written by men that it is the record of work carried out by me and that it has not been submitted in any previous application for a higher degree. ý.. 'C) : %6 date .... .... signature of candidat 1404100 I was admitted as a research student under Ordinance No. 12 on I October 1977 and as a candidate for the degree of Ph. D. on I October 1978; the higher study for which this is a record was carried out in the University of St Andrews between 1977 and 1980. $'ý.... date . .. 0&0.9 0. signature of candidat I hereby certify that the candidate has fulfilled the conditions of the Resolution and Regulations appropriate to the degree of Ph. D. of the University of St Andrews and that he is qualified to submit this thesis in application for that degree. -

110508NU-Ep511-Yellow Script
“Arrow of Time” #511/Ep. 90 Written by Ken Sanzel Directed by Ken Sanzel Production Draft – 10/17/08 Rev. FULL Blue – 10/30/08 Rev. Pink – 11/4/08 Rev. Yellow – 11/5/08 (Pages: 41,46,50,55.) SCOTT FREE in association with CBS PARAMOUNT NETWORK TELEVISION, a division of CBS Studios. © Copyright 2008 CBS Paramount Network Television. All Rights Reserved. This script is the property of CBS Paramount Network Television and may not be copied or distributed without the express written permission of CBS Paramount Network Television. This copy of the script remains the property of CBS Paramount Network Television. It may not be sold or transferred and must be returned to: CBS Paramount Network Television Legal Affairs 4024 Radford Avenue Administration Bldg., Suite 390, Studio City, CA 91604 THE WRITING CREDITS MAY OR MAY NOT BE FINAL AND SHOULD NOT BE USED FOR PUBLICITY OR ADVERTISING PURPOSES WITHOUT FIRST CHECKING WITH TELEVISION LEGAL DEPARTMENT. “Arrow of Time” Ep. #511 – Production Draft: Rev. Yellow – 11/5/08 SCRIPT REVISION HISTORY COLOR DATE PAGES WHITE 10/17/08 (1-57) REV. FULL BLUE 10/30/08 (1-57) REV. PINK 11/4/08 (2,3,4,6,7,18,19,20,27,33, 34,39,41,42,49,50,51,55.) REV. YELLOW 11/5/08 (41,46,50,55.) “Arrow of Time” Ep. #511 – Production Draft: Rev. FULL Blue – 10/30/08 CAST LIST DON EPPES CHARLIE EPPES ALAN EPPES DAVID SINCLAIR LARRY FLEINHARDT AMITA RAMANUJAN COLBY GRANGER NIKKI BETANCOURT LIZ WARNER ROBIN BROOKS BUCK WINTERS RAFE LANSKY GRAY McCLAUGHLIN JOE THIBODEAUX * DEANNE DRAKE TOBY TIM PYNCHON SECOND MARSHAL “Arrow of Time” Ep. -

Numb3rs Episode Guide Episodes 001–118
Numb3rs Episode Guide Episodes 001–118 Last episode aired Friday March 12, 2010 www.cbs.com c c 2010 www.tv.com c 2010 www.cbs.com c 2010 www.redhawke.org c 2010 vitemo.com The summaries and recaps of all the Numb3rs episodes were downloaded from http://www.tv.com and http://www. cbs.com and http://www.redhawke.org and http://vitemo.com and processed through a perl program to transform them in a LATEX file, for pretty printing. So, do not blame me for errors in the text ^¨ This booklet was LATEXed on June 28, 2017 by footstep11 with create_eps_guide v0.59 Contents Season 1 1 1 Pilot ...............................................3 2 Uncertainty Principle . .5 3 Vector ..............................................7 4 Structural Corruption . .9 5 Prime Suspect . 11 6 Sabotage . 13 7 Counterfeit Reality . 15 8 Identity Crisis . 17 9 Sniper Zero . 19 10 Dirty Bomb . 21 11 Sacrifice . 23 12 Noisy Edge . 25 13 Man Hunt . 27 Season 2 29 1 Judgment Call . 31 2 Bettor or Worse . 33 3 Obsession . 37 4 Calculated Risk . 39 5 Assassin . 41 6 Soft Target . 43 7 Convergence . 45 8 In Plain Sight . 47 9 Toxin............................................... 49 10 Bones of Contention . 51 11 Scorched . 53 12 TheOG ............................................. 55 13 Double Down . 57 14 Harvest . 59 15 The Running Man . 61 16 Protest . 63 17 Mind Games . 65 18 All’s Fair . 67 19 Dark Matter . 69 20 Guns and Roses . 71 21 Rampage . 73 22 Backscatter . 75 23 Undercurrents . 77 24 Hot Shot . 81 Numb3rs Episode Guide Season 3 83 1 Spree ............................................. -

A Panacea for Local Government? the Role of PR
A Panacea for Local Government? The Role of PR David Sinclair Research Fellow October 1998 Constitution Unit publications can be ordered from the following address: The Constitution Unit School of Public Policy 29/30 Tavistock Square London WC 1H 9EZ Tel: 0 17 1 504 4977 Fax: 0 17 1 504 4978 Email: [email protected] CONTENTS Executive Summary 3 Introduction 4 Representation of the Citizen 5 Substantive representation 6 The end of one-party states 7 Descriptive representation 8 Responsiveness and Accountability of Local Government 9 Constituency links 9 PR and annual elections 10 Effective Decision Making 12 Turnout in Local Elections 16 Conclusion 18 Appendix A - PR and the Representation of Women 19 Appendix B - The Main Electoral Systems 22 Appendix C - One-Party States 24 Executive Summary With proportional representation (PR) being introduced into Britain at the regional, European and possibly national level, there is increasing interest in the role that PR might play in revitalising local democracy. This Briefing analyses the likely effects of introducing PR for local government elections, and the compatibility of PR with the rest of the government's agenda for modernising local government. The main findings are: Compared to the existing first past the post system, PR wiU increase party competition and opposition representation in local government and will reduce the incidence of one-party states. Most forms of PR reduce the constituency/member link; but the impact of this reduction would be less marked than introducing PR at a national level, because most local government wards already have more than one councillor. -

Leni Sinclair Is the 2016 Kresge Eminent Artist
2016 KRESGE EMINENT ARTIST LENI SINC LAIR The Kresge Eminent Artist Award honors an exceptional artist in the visual, performing or literary arts for lifelong profes- sional achievements and contributions to metropolitan Detroit’s cultural community. | Leni Sinclair is the 2016 Kresge Eminent Artist. This monograph commemorates her life and work. Fred “Sonic” Smith in concert with the MC5 at Michigan State University, Lansing, Michigan, 1969. Front Cover: Dallas Hodge Band concert at Gallup Park, Ann Arbor, Michigan, 1974. 5 Forward Other Voices 42 The Greatness 76 “John Sinclair” Kresge Arts in Detroit By Rip Rapson 13 Hugh “Buck” Davis of Leni Sinclair By John Lennon 108 2015-16 Kresge Arts in Detroit President and CEO 27 Bill Harris By John Sinclair Advisory Council The Kresge Foundation 35 Robin Eichele 78 A People’s History of the 47 Harvey Ovshinsky CIA Bombing Conspiracy 108 The Kresge Eminent Artist 6 Artist’s Statement 55 Peter Werbe Art (The Keith Case): Or, Award and Winners 71 Juanita Moore and Lars Bjorn 52 When Photography How the White Panthers 85 Judge Damon J. Keith is Revolution: Saved the Movement 110 About The Kresge Foundation Life 97 Rebecca Derminer Notes on Leni Sinclair By Hugh “Buck” Davis Board of Trustees 10 Leni Sinclair: Back 97 Barbara Weinberg Barefield By Cary Loren Credits In The Picture 90 Photographs: Acknowledgements By Sue Levytsky 62 Leni Sinclair: The Times 40 Coming to Amerika: Out of the Dark 22 A Reader’s Guide Leni Sinclair Chronicled Our By Herb Boyd 100 Biography Dreams and Aspirations 24 Photographs: By George Tysh 107 Our Congratulations Michelle Perron The Music Activism 68 The Evolution of Director, Kresge Arts in Detroit a Commune 107 A Note From By Leni Sinclair Richard L. -

Individual and Organizational Donors
INDIVIDUAL AND ORGANIZATIONAL Mr. Saumya Nandi and Ms. Martha Delgado Edward & Rose Donnell Foundation Dr. Tim D. Noel and Mrs. Joni L. Noel Mr. and Mrs. John A. Edwardson DONORS Orange Crush, LLC Ms. Amberlynne Farashahi Park Avenue Financial Group Trust Mr. and Mrs. Blair Farwell $100,000 and above Mr. and Mrs. Mark J. Parrell The Field Foundation of Illinois Anonymous (4) The Pritzker Pucker Family Foundation Fortune Brands, Inc. Bank of America Mr. Richard Proulx Franklin Philanthropic Foundation BlackEdge Capital Bruce and Diana Rauner Mr. Philip M. Friedmann The Chicago Community Trust The Regenstein Foundation Futures Industry Association Feeding America Mr. and Mrs. Bradley S. Reid Garvey's Office Products Ms. Susan E. Grabin The Rhoades Foundation GCA Services Group, Inc. Hardison Family Foundation Mr. and Mrs. James H. Roth General Iron Industries Charitable Foundation Mr. and Mrs. Raymond L. Harriman Roundy's Foundation Dr. Glenn S. Gerber and Ms. Linda S. Schurman Hillshire Brands Foundation The Satter Family Foundation Gethsemane United Church of Christ Daniel Haerther Living Trust Mr. and Mrs. Travis Schuler Mr. and Mrs. Brent Gledhill Mr. Albert F. Hofeld Mrs. Rose L. Shure Goldberg Kohn, Ltd. Mr. Michael L. Keiser and Mrs. Rosalind Keiser Julie and Brian Simmons Foundation Golub & Company Kraft Foods Group Foundation SmithBucklin Corporation Google, Inc. Ann Lurie Revocable Trust The Smogolski Family 2008 Mr. and Mrs. Andrew M. Gore Polk Bros. Foundation Charitable Lead Trust W.W. Grainger, Inc. Share Our Strength The Telos Group LLC Grand Kids Foundation Mr. William R. Shepard Stanley and Lucy Lopata Charitable Foundation Ms. -

102808NU-Ep510-Goldenrod Script
“Frienemies” #510/Ep. 89 Written by Cheryl Heuton & Nicolas Falacci Directed by Steve Boyum Production Draft – 10/10/08 Rev. Blue – 10/15/08 Rev. FULL Pink – 10/20/08 Rev. Yellow – 10/23/08 Rev. Green – 10/24/08 Rev. Goldenrod – 10/28/08 (Pages: 32,36.) SCOTT FREE in association with CBS PARAMOUNT NETWORK TELEVISION, a division of CBS Studios. © Copyright 2008 CBS Paramount Network Television. All Rights Reserved. This script is the property of CBS Paramount Network Television and may not be copied or distributed without the express written permission of CBS Paramount Network Television. This copy of the script remains the property of CBS Paramount Network Television. It may not be sold or transferred and must be returned to: CBS Paramount Network Television Legal Affairs 4024 Radford Avenue Administration Bldg., Suite 390, Studio City, CA 91604 THE WRITING CREDITS MAY OR MAY NOT BE FINAL AND SHOULD NOT BE USED FOR PUBLICITY OR ADVERTISING PURPOSES WITHOUT FIRST CHECKING WITH TELEVISION LEGAL DEPARTMENT. “Frienemies” Ep. #510 – Production Draft: Rev. Goldenrod – 10/28/08 SCRIPT REVISION HISTORY COLOR DATE PAGES WHITE 10/10/08 (1-55) REV. BLUE 10/15/08 (1,2,4,5,8,12,14,19,21, 22,26,27,29,31,33,35, 37,41.) REV. FULL PINK 10/20/08 (1-56) REV. YELLOW 10/23/08 (1,2,3,6,8,13,15,16,18, 19,20,28,34,36,38,39, 40,40A,47,48,54,55.) REV. GREEN 10/24/08 (14,34,36,55.) REV. GOLDENROD 10/28/08 (32,36.) “Frienemies” Ep. -

Download Full Episodes of Numb3rs Online Free Numb3rs (TV Series) on May 18, 2010, CBS Announced That Numbers Had Been Cancelled After Six Seasons
download full episodes of numb3rs online free Numb3rs (TV series) On May 18, 2010, CBS announced that Numbers had been cancelled after six seasons. Contents. Summary [ edit | edit source ] The show focuses equally on the relationships among Don Eppes, his brother Charlie Eppes, and their father, Alan Eppes (Judd Hirsch), and on the brothers' efforts to fight crime, usually in Los Angeles. A typical episode begins with a crime, which is subsequently investigated by a team of FBI agents led by Don and mathematically modeled by Charlie, with the help of Larry Fleinhardt (Peter MacNicol) and Amita Ramanujan (Navi Rawat). The insights provided by Charlie's mathematics were always in some way crucial to solving the crime. Cast and characters [ edit | edit source ] as Don Eppes as Charlie Eppes as Alan Eppes as David Sinclair as Terry Lake (Season 1) as Colby Granger as Megan Reeves (Season 2–4) as Amita Ramanujan (Season 2–6, recurring Season 1) as Nikki Betancourt (Season 5–6) as Liz Warner (Season 5–6, recurring Season 3–4) as Larry Fleinhardt. Episodes [ edit | edit source ] Main article: Episode Guide Season Episodes Originally aired Season premiere Season finale 1 13 January 23, 2005 May 13, 2005 2 24 September 23, 2005 May 19, 2006 3 24 September 22, 2006 May 18, 2007 4 18 September 28, 2007 May 16, 2008 5 23 October 3, 2008 May 15, 2009 6 16 September 25, 2009 March 12, 2010. Production [ edit | edit source ] The idea for Numbers was generated in the late 1990s when Nick Falacci and Cheryl Heuton, the show's creators, attended a lecture given by Bill Nye, a popular science educator. -

Book Reviews
244 BOOK REVIEWS Geography and History. Bridging the Divide. ALAN R.H. BAKER. Cambridge: Cambridge University Press, 2003, Pp. vii+227, maps, illustrations, diagrams, index. $25.99 paper. ISBN 0521288851. Historical geographer, Alan R.H. Baker, provides a comprehensive, interdis- ciplinary, and multinational approach to understanding the interrelationships of geography and history. He seeks to contribute “a common language in which [geographers and historians] can conduct meaningful dialogues” (p. 2). Baker approaches the task of providing a book-length treatment of the connections between geography and history from the perspectives of both disciplines as well as through the lenses of historical geography and geographical history. By explor- ing their “‘spaces of contact,’ he offers a framework for ‘bridging the divide’ be- tween geography and history.” Many geographers, historians, and historical geographers in particular al- ready recognize and appreciate the connections and shared systems of meaning between the two fields. For this group, the book reinforces their perceptions with numerous examples of works that incorporate both geographic and historical methodologies and perspectives. This study will also “be of interest more gener- ally both to historians seeking more knowledge and understanding of the ideas and practices of geographers and to geographers wishing to improve their knowl- edge and understanding of the ideas and practices of historians” (p. 2). It is per- haps, this group that lacks a clear understanding of the interconnectedness of history and geography, which serves as the primary target of this book. More specifically, Baker explains that he designed this book for advanced undergradu- ate or graduate students in both geography and history (p. -

House Research Organization • Texas House of Representatives P.O
HOUSE RESEARCH ORGANIZATION • TEXAS HOUSE OF REPRESENTATIVES P.O. Box 2910, Austin, Texas 78768-2910 (512) 463-0752 • http://www.hro.house.state.tx.us Steering Committee: Alma Allen, Chairman Dwayne Bohac, Vice Chairman Rafael Anchia Donna Howard Eddie Lucio III Myra Crownover Joe Farias Bryan Hughes Susan King Doug Miller Joe Deshotel John Frullo Ken King J. M. Lozano Joe Pickett HOUSE RESEARCH ORGANIZATION daily floor report Tuesday, April 28, 2015 84th Legislature, Number 58 The House convenes at 10 a.m. Eighteen bills are on the daily calendar for second-reading consideration today. The bills analyzed or digested are listed on the following page. Alma Allen Chairman 84(R) - 58 HOUSE RESEARCH ORGANIZATION Daily Floor Report Tuesday, April 28, 2015 84th Legislature, Number 58 HB 2154 by Dutton Sunset review of the State Office of Administrative Hearings 1 HB 31 by Bonnen Decreasing the state sales tax rate 10 HB 32 by Bonnen Reducing franchise tax rate and expanding E-Z computation eligibility 16 HB 559 by Anchia Telling arrestees of immigration consequences of guilty, no contest pleas 22 HB 364 by Dutton Creating an affirmative defense in enforcement of child support actions 26 HB 3373 by Miller Liability of employers reimbursing TWC for unemployment benefits 29 HB 3052 by Bonnen Allocating a portion of the hotel occupancy tax to certain municipalities 32 HB 2753 by Villalba Changing the standard for approving names of certain businesses 34 HB 2400 by Bohac Exempting certain motor vehicle transfers from the sales tax 38 HB 2115 by Phillips Extending the initial two-year inspection period to certain fleet vehicles 41 HB 1550 by Zerwas Ability of pharmacists to administer epinephrine 43 HB 1887 by Muñoz Establishing a center for public safety training in the Rio Grande Valley 46 HB 606 by Davis Requiring a study utilizing prenatal surgery to treat birth defects. -

How Democratic Is the American Constitution?
00dahlFM.i_x 11/27/01 4:37 PM Page i The Castle Lectures in Ethics, Politics, and Economics 00dahlFM.i_x 11/27/01 4:37 PM Page ii 00dahlFM.i_x 11/27/01 4:37 PM Page iii How Democratic Is the American Constitution? Robert A. Dahl Yale University Press / New Haven & London 00dahlFM.i_x 11/27/01 4:37 PM Page iv Published with assistance from the foundation established in memory of Philip Hamilton McMillan of the Class of 1894, Yale College. Copyright © 2001 by Yale University. All rights reserved. This book may not be reproduced, in whole or in part, including illustrations, in any form (beyond that copying permitted by Sections 107 and 108 of the U.S. Copyright Law and except by reviewers for the public press), without written permission from the publishers. Designed by James J. Johnson and set in New Caledonia and Bulmer types by Integrated Publishing Solutions. Printed in the United States of America. Library of Congress Cataloging-in-Publication Data Dahl, Robert Alan, 1915– How democratic is the American Constitution?/ Robert A. Dahl. p. cm. — (The Castle lecture series) Includes bibliographical references and index. ISBN 0-300-09218-0 (cloth : alk. paper) 1. Constitutional law—United States. 2. Constitutional history— United States. 3. Democracy. I. Title. II. Series. KF4550 .D34 2001 342.73’02—dc21 2001004323 A catalogue record for this book is available from the British Library. The paper in this book meets the guidelines for permanence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources.