Vectura Group Plc Report and Accounts for the Nine-Month Period
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jefferies 2013 Global Healthcare Conference in London
Jefferies 2013 Global Healthcare Conference in London At the 2012 Jefferies Global Healthcare Conference, there were well over 200 healthcare companies participating with a combined market cap of $1 trillion and close to 1,300 one-on-one/small group meetings over the two-day event. The 2013 conference is set to follow the same footsteps, featuring public and private leading INVITATION global healthcare companies within the areas of pharmaceuticals, biotech, generics, 20-21 NOVEMBER 2013 medtech and healthcare services from the US, Central and Eastern Europe, Latin The Waldorf Hilton America, India, China, Japan, Egypt, Israel and Russia. London, UK Throughout the two-day event, we will feature concurrent tracks of informative presentations as well as 1x1/small group meetings, and thematic panel discussions. This global gathering of institutional investors, private equity investors, VCs and leading executives will address near- and long-term investment opportunities and discuss the mechanisms driving global healthcare. We hope you can join us for what promises to be a unique and comprehensive view of the industry. Registration is now open. Please email your Jefferies representative if you are interested in attending. For general questions, please email [email protected] or contact your Jefferies representative. © 2013 Jefferies LLC. Member SIPC. AGENDA WEDNESDAY, 20 NOVEMBER 2013 Jefferies 2013 Global Healthcare Conference in London ADELPHI 1 ADELPHI 2 ADELPHI 3 EXECUTIVE BOARDROOM 7:30 AM Breakfast & Registration 8:00 AM Clinigen Group Plc Ion Beam Applications Active Biotech AB Syneron Medical Ltd. Specialty Pharma & Healthcare Services Medical Products Biotechnology Medical Technology Paul Thomas; CTO Olivier Legrain, CEO Tomas Leanderson; President and CEO Hugo Goldman, CFO 8:40 AM Valneva Celltrion, Inc. -

Retirement Strategy Fund 2060 Description Plan 3S DCP & JRA
Retirement Strategy Fund 2060 June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA ACTIVIA PROPERTIES INC REIT 0.0137% 0.0137% AEON REIT INVESTMENT CORP REIT 0.0195% 0.0195% ALEXANDER + BALDWIN INC REIT 0.0118% 0.0118% ALEXANDRIA REAL ESTATE EQUIT REIT USD.01 0.0585% 0.0585% ALLIANCEBERNSTEIN GOVT STIF SSC FUND 64BA AGIS 587 0.0329% 0.0329% ALLIED PROPERTIES REAL ESTAT REIT 0.0219% 0.0219% AMERICAN CAMPUS COMMUNITIES REIT USD.01 0.0277% 0.0277% AMERICAN HOMES 4 RENT A REIT USD.01 0.0396% 0.0396% AMERICOLD REALTY TRUST REIT USD.01 0.0427% 0.0427% ARMADA HOFFLER PROPERTIES IN REIT USD.01 0.0124% 0.0124% AROUNDTOWN SA COMMON STOCK EUR.01 0.0248% 0.0248% ASSURA PLC REIT GBP.1 0.0319% 0.0319% AUSTRALIAN DOLLAR 0.0061% 0.0061% AZRIELI GROUP LTD COMMON STOCK ILS.1 0.0101% 0.0101% BLUEROCK RESIDENTIAL GROWTH REIT USD.01 0.0102% 0.0102% BOSTON PROPERTIES INC REIT USD.01 0.0580% 0.0580% BRAZILIAN REAL 0.0000% 0.0000% BRIXMOR PROPERTY GROUP INC REIT USD.01 0.0418% 0.0418% CA IMMOBILIEN ANLAGEN AG COMMON STOCK 0.0191% 0.0191% CAMDEN PROPERTY TRUST REIT USD.01 0.0394% 0.0394% CANADIAN DOLLAR 0.0005% 0.0005% CAPITALAND COMMERCIAL TRUST REIT 0.0228% 0.0228% CIFI HOLDINGS GROUP CO LTD COMMON STOCK HKD.1 0.0105% 0.0105% CITY DEVELOPMENTS LTD COMMON STOCK 0.0129% 0.0129% CK ASSET HOLDINGS LTD COMMON STOCK HKD1.0 0.0378% 0.0378% COMFORIA RESIDENTIAL REIT IN REIT 0.0328% 0.0328% COUSINS PROPERTIES INC REIT USD1.0 0.0403% 0.0403% CUBESMART REIT USD.01 0.0359% 0.0359% DAIWA OFFICE INVESTMENT -

Cboe UK Healthcare Sector Index BUKHLTH
Cboe UK Healthcare Sector Index BUKHLTH Page 1 August 2021 Cboe Exchange This sector represents products and services that are designed, developed, and utilized in the promotion of health and well-being, including medical services, health plans, medical devices, and biopharmaceuticals. The parent index is the Cboe UK All Companies (BUKAC). The index base level is 10,000 as of December 31st, 2010. This is a price return index. Objective The index is designed for use in the creation of index tracking funds, derivatives and as a performance benchmark. Investability Liquidity Transparency Availability Stocks are selected and Stocks are screened to Uses a transparent, rules-based Calculation is based on weighted to ensure that the ensure that the index is construction process. Index price and total return index is investable. tradable. Rules are freely available on the methodologies, both real cboe.com/europe/indices -time, intra-second and website. end of day. Statistics Index ISIN Ticker RIC Currency Cboe UK Healthcare Sector DE000SLA1DJ6 BUKHLTH .BUKHLTH GBP Cboe UK Healthcare Sector - net DE000SLA18F5 BUKHLTHN .BUKHLTHN GBP Volatility Volatility (1y) 0.1776 Returns(%) 1M 3M 6M YTD 1Y 3Y 5Y BUKHLTH 2.03 2.35 16.31 7.02 -2.85 13.78 17.14 BUKHLTHN 2.97 3.33 18.35 10.28 0.52 26.24 39.74 Top 5 Performers Country 1 month return % INDIVIOR PLC UNITED KINGDOM 17.70 MEDICLINIC INTERNATIONAL PLC UNITED KINGDOM 12.80 OXFORD BIOMEDICA PLC UNITED KINGDOM 11.38 PURETECH HEALTH PLC UNITED KINGDOM 8.77 VECTURA GROUP PLC UNITED KINGDOM 7.76 Historical Performance Chart 100% 80% 60% 40% 20% 0% 2011 2013 2014 2016 2017 2018 2020 2021 Cboe UK Healthcare Sector (GBP) Cboe UK All Companies (GBP) Cboe.com | ©Cboe | /CboeGlobalMarkets | /company/cboe © 2021 Cboe Exchange, Inc. -

Portfolio Holdings Listing Fidelity International Small Cap Opportunities Fund DUMMY As of June 30, 2021
Portfolio Holdings Listing Fidelity International Small Cap Opportunities Fund DUMMY as of June 30, 2021 The portfolio holdings listing (listing) provides information on a fund’s investments as of the date indicated. Top 10 holdings information (top 10 holdings) is also provided for certain equity and high income funds. The listing and top 10 holdings are not part of a fund’s annual/semiannual report or Form N-Q and have not been audited. The information provided in this listing and top 10 holdings may differ from a fund’s holdings disclosed in its annual/semiannual report and Form N-Q as follows, where applicable: With certain exceptions, the listing and top 10 holdings provide information on the direct holdings of a fund as well as a fund’s pro rata share of any securities and other investments held indirectly through investment in underlying non- money market Fidelity Central Funds. A fund’s pro rata share of the underlying holdings of any investment in high income and floating rate central funds is provided at a fund’s fiscal quarter end. For certain funds, direct holdings in high income or convertible securities are presented at a fund’s fiscal quarter end and are presented collectively for other periods. For the annual/semiannual report, a fund’s investments include trades executed through the end of the last business day of the period. This listing and the top 10 holdings include trades executed through the end of the prior business day. The listing includes any investment in derivative instruments, and excludes the value of any cash collateral held for securities on loan and a fund’s net other assets. -

20 Years of Strategic Growth
Dechra Pharmaceuticals PLC Annual Report and Accounts for the year ended 30 June 2017 20 Years of Strategic Growth ® Stock Code: DPH Annual Report and Accounts for the year ended 30 June 2017 Company Number: 3369634 slugline Dechra Annual Report 2017 - Front.indd 3 slugline 9/14/2017 8:32:51 AM Welcome to Dechra Pharmaceuticals PLC Dechra is an international specialist veterinary pharmaceuticals and related products business. Our expertise is in the development, manufacture, and sales and marketing of high quality products exclusively for veterinarians worldwide. Getting Around the Report Forward-Looking Statements Icons are used within the Report to assist the reader to identify This document contains certain forward-looking statements. links to other relevant sections of interest. Below is a selection The forward-looking statements reflect the knowledge and of icons you will see used within this Report: information available to the Company during preparation and up to the publication of this document. By their very nature, For more information see further pages these statements depend upon circumstances and relate to within the Report events that may occur in the future and thereby involve a degree of uncertainty. Therefore, nothing in this document should be construed as a profit forecast by the Company. More information online at: www.dechra.com Glossary Terms used within this section Open the flaps to view Our History Dechra Annual Report 2017 - Front.indd 4 slugline 04/09/2017 13:58:27 Delivering Consistently for Our Shareholders Dechra is a company that has consistently delivered on its strategic objectives resulting in a strong track record of growth. -

FTSE Publications
2 FTSE Russell Publications FTSE Developed Europe SMID Cap 19 August 2021 Tradable Plus Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) 3i Group 0.72 UNITED Bureau Veritas S.A. 0.42 FRANCE Gjensidige Forsikring ASA 0.17 NORWAY KINGDOM Campari 0.31 ITALY Gn Store Nordic 0.53 DENMARK AAK 0.18 SWEDEN Carl Zeiss Meditec 0.33 GERMANY Halma 0.66 UNITED Aalberts NV 0.24 NETHERLANDS Castellum 0.32 SWEDEN KINGDOM ABN AMRO Bank NV 0.23 NETHERLANDS Centrica 0.2 UNITED Hargreaves Lansdown 0.39 UNITED Acciona S.A. 0.16 SPAIN KINGDOM KINGDOM Accor 0.32 FRANCE Chr. Hansen Holding A/S 0.43 DENMARK Hays 0.16 UNITED ACS Actividades Cons y Serv 0.3 SPAIN Clariant 0.21 SWITZERLAND KINGDOM Adecco Group AG 0.49 SWITZERLAND Coca-Cola HBC AG 0.33 UNITED HeidelbergCement AG 0.6 GERMANY Adevinta 0.21 NORWAY KINGDOM HelloFresh SE 0.67 GERMANY Admiral Group 0.43 UNITED Cofinimmo 0.21 BELGIUM Helvetia Holding AG 0.18 SWITZERLAND KINGDOM Commerzbank 0.35 GERMANY Hikma Pharmaceuticals 0.27 UNITED Aedifica 0.22 BELGIUM ConvaTec Group 0.25 UNITED KINGDOM Aegon NV 0.34 NETHERLANDS KINGDOM Hiscox 0.18 UNITED KINGDOM Aeroports de Paris 0.17 FRANCE Countryside Properties 0.16 UNITED Holmen AB 0.2 SWEDEN Ageas 0.46 BELGIUM KINGDOM Homeserve 0.17 UNITED Aker BP ASA 0.16 NORWAY Covestro AG 0.54 GERMANY KINGDOM Alfa Laval 0.52 SWEDEN Covivio 0.2 FRANCE Howden Joinery Group 0.3 UNITED Alstom 0.7 FRANCE Croda International 0.64 UNITED KINGDOM KINGDOM Alten 0.18 -

Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Brown Capital Management International Small Company Fund Schedule of Investments As of December 31, 2020 (Unaudited)
7 Brown Capital Management International Small Company Fund Schedule of Investments As of December 31, 2020 (Unaudited) Shares Value (Note 1) COMMON STOCKS - 96.60% Australia - 6.06% 708,305 REA Group, Ltd. $ 81,287,705 1,464,269 WiseTech Global, Ltd. 34,713,030 116,000,735 Austria - 0.72% 364,651 Schoeller-Bleckmann Oilfield Equipment AG 13,854,217 Canada - 8.81% (a) 1,493,328 Descartes Systems Group, Inc. 87,342,501 (a) 574,515 Kinaxis, Inc. 81,395,267 168,737,768 Denmark - 8.36% 1,718,159 Ambu A/S - Class B 74,226,815 1,231,585 NNIT A/S 24,581,567 412,166 SimCorp A/S 61,259,325 160,067,707 France - 10.09% 1,499,660 Albioma SA 86,106,283 12,180 Esker SA 2,627,739 1,167,698 Interparfums SA 61,268,589 1,412,713 Lectra 43,145,760 193,148,371 Germany - 13.03% (a) 2,470,144 Evotec SE 91,373,934 1,216,343 Nexus AG 75,782,759 548,366 STRATEC SE 82,264,614 249,421,307 Hong Kong - 3.55% 16,666,000 Kingdee International Software Group Co., Ltd. 67,932,795 India - 1.37% 588,229 CRISIL, Ltd. 15,469,415 1,862,265 Emami, Ltd. 10,800,079 26,269,494 Ireland - 1.34% 125,371 Flutter Entertainment PLC 25,562,163 Israel - 3.46% (a) 410,426 CyberArk Software, Ltd. 66,320,737 Italy - 1.62% 1,424,162 Azimut Holding SpA 30,916,548 Japan - 12.95% 245,400 GMO Payment Gateway, Inc. -

Coleman Street Investments
1 | COLEMAN STREET INVESTMENTS COLEMAN STREET INVESTMENTS A Luxembourg domiciled Open-Ended Investment Fund With multiple sub-funds Annual Report Including audited Financial Statements st As of 31 December, 2018 Luxembourg Corporate and Trade Register RCS No B175-811 No subscriptions can be made on the basis of this annual report including audited annual financial statements. Subscriptions are only valid if they are made on the basis of the latest prospectus accompanied by the last Annual Report including Audited Financial Statements and the last unaudited semi-annual Report, if the latter is more recent than the Annual Report including audited financial statements. The subscription, conversion and repurchasing forms can be obtained on request addressed to the Registered Office of the Company at 287, Route d’Arlon, L-1150 Luxembourg, G.D.L. 2 | COLEMAN STREET INVESTMENTS TABLE OF CONTENTS ORGANISATION AND ADMINISTRATION ................................................................................................................................................. 3 MANAGEMENT REPORT ........................................................................................................................................................................... 6 REPORT OF THE REVISEUR D’ENTREPRISES AGREE .......................................................................................................................... 9 STATEMENT OF NET ASSETS ............................................................................................................................................................... -

2020 Half Year Report
MidCap_HY_Cover.qxp 25/02/2021 11:25 Page FC1 JPMorgan Mid Cap Investment Trust plc Half Year Report & Financial Statements for the six months ended 31st December 2020 MidCap_HY_Cover.qxp 25/02/2021 11:25 Page IFC2 KEY FEATURES Your Company Objective JPMorgan Mid Cap Investment Trust plc (the ‘Company’) aims to achieve capital growth from investment in medium-sized UK listed companies. The Company specialises in investment in FTSE 250 companies, using long and short term borrowings to increase returns to shareholders. Investment Policies • To focus on FTSE 250 stocks that deliver strong capital growth. • To have significant exposure to the UK economy, with selective exposure to overseas earnings. • To seek out both value stocks and growth stocks, including AIM stocks, to deliver strong performance throughout the market cycle. • To use gearing to increase potential returns to shareholders. • To invest no more than 15% of gross assets in other UK listed investment companies (including investment trusts). The Company’s shares are designed for private investors in the UK, including retail investors, professionally-advised private clients and institutional investors, who seek the potential for capital growth from investment in the UK market and who understand and are willing to accept the risks of exposure to equities. Private investors may wish to consider consulting an independent financial adviser who specialises in advising on the acquisition of shares and other securities before acquiring shares in the Company. Investors should be capable of evaluating the risks and merits of such an investment and should have sufficient resources to bear any loss that may result. -

FTSE Russell Publications
2 FTSE Russell Publications 19 August 2021 FTSE 250 Indicative Index Weight Data as at Closing on 30 June 2021 Index weight Index weight Index weight Constituent Country Constituent Country Constituent Country (%) (%) (%) 3i Infrastructure 0.43 UNITED Bytes Technology Group 0.23 UNITED Edinburgh Investment Trust 0.25 UNITED KINGDOM KINGDOM KINGDOM 4imprint Group 0.18 UNITED C&C Group 0.23 UNITED Edinburgh Worldwide Inv Tst 0.35 UNITED KINGDOM KINGDOM KINGDOM 888 Holdings 0.25 UNITED Cairn Energy 0.17 UNITED Electrocomponents 1.18 UNITED KINGDOM KINGDOM KINGDOM Aberforth Smaller Companies Tst 0.33 UNITED Caledonia Investments 0.25 UNITED Elementis 0.21 UNITED KINGDOM KINGDOM KINGDOM Aggreko 0.51 UNITED Capita 0.15 UNITED Energean 0.21 UNITED KINGDOM KINGDOM KINGDOM Airtel Africa 0.19 UNITED Capital & Counties Properties 0.29 UNITED Essentra 0.23 UNITED KINGDOM KINGDOM KINGDOM AJ Bell 0.31 UNITED Carnival 0.54 UNITED Euromoney Institutional Investor 0.26 UNITED KINGDOM KINGDOM KINGDOM Alliance Trust 0.77 UNITED Centamin 0.27 UNITED European Opportunities Trust 0.19 UNITED KINGDOM KINGDOM KINGDOM Allianz Technology Trust 0.31 UNITED Centrica 0.74 UNITED F&C Investment Trust 1.1 UNITED KINGDOM KINGDOM KINGDOM AO World 0.18 UNITED Chemring Group 0.2 UNITED FDM Group Holdings 0.21 UNITED KINGDOM KINGDOM KINGDOM Apax Global Alpha 0.17 UNITED Chrysalis Investments 0.33 UNITED Ferrexpo 0.3 UNITED KINGDOM KINGDOM KINGDOM Ascential 0.4 UNITED Cineworld Group 0.19 UNITED Fidelity China Special Situations 0.35 UNITED KINGDOM KINGDOM KINGDOM Ashmore -
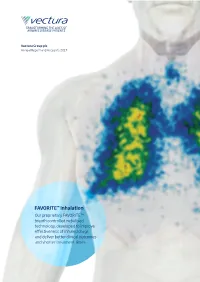
Vectura Group Plc Annual Report and Accounts 2017 Annual Report and Accounts 2017 Accounts and Report Annual
TRANSFORMING THE LIVES OF AIRWAYS DISEASE PATIENTS Vectura Group plc Vectura Group plc Annual Report and Accounts 2017 Annual Report and Accounts 2017 FAVORITE™ inhalation Our proprietary FAVORITE™ breath controlled nebulised technology, developed to improve effectiveness of inhaled drugs and deliver better clinical outcomes and shorter treatment times ASTHMA Asthma is one of the most common non-communicable diseases.1 Asthma is a chronic, reversible disease that inflames and narrows airways in the lungs, causing wheezing, chest tightness and coughing.2 Not all asthma is the same and severe asthma can have a number of underlying causes.3 Triggers4 Dust mites Pets Sulphites in Infections, such Physical activity, foods and drinks as colds including exercise Symptoms Prevalence • Recurring attacks of breathlessness and wheezing that • Asthma is a significant health burden of considerable vary in severity and frequency from person to person5 and growing significance with increasing urbanisation7 • Asthma attacks – a sudden worsening of symptoms, • Estimated 10m adults under the age of 45 affected in Europe9 can be unpredictable and 18.4m adults in the US9 • During an asthma attack, the lining of the bronchial • Prevalence continues to increase and it is estimated that tubes swell, causing the airways to narrow and by 2025 asthma will affect more than 400m people globally10 6 reducing the flow of air into and out of the lungs • Asthma affects people of all ages but most frequently begins in childhood11 14% of the world’s children experience asthma symptoms12 Impact 40–50% • Patients with uncontrolled asthma are significantly of patients are not well controlled14; poor more likely to suffer from poor outcomes and adherence to treatment and device user errors contribute to lack of control 15 medical emergencies • Asthma caused 383,000 deaths in 201513 By 2025 the total asthma market is expected to be worth over $17bn16 1 Latest WHO estimates, released in December 2016, 9 2014; 12; 24: 14009; Centers for Disease, Control and Prevention (CDC).