Non-Reproductive Seasonal Colour Change in a Population of Calotes “Versicolor” from Myanmar (Squamata: Agamidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BIB 13484.Pdf
Russian Journal of Herpetology Vol. 26, No. 5, 2019, pp. 247 – 260 DOI: 10.30906/1026-2296-2019-26-5-247-260 REAPPRAISAL OF HERPETOFAUNA RECORDED FROM JAFFNA PENINSULA IN NORTHERN SRI LANKA WITH REMARKS ON CONSERVATION, DIVERSITY, AND DISTRIBUTION Majintha Madawala,1 Thilina Surasinghe,2* Anslem De Silva,3 Dinesh Gabadage,4 Madhava Botejue,4 Indika Peabotuwage,5 Dushantha Kandambi,5 and Suranjan Karunarathna5 Submitted January 11, 2017 Jaffna peninsula is quite an unexplored area of Sri Lanka’s lowland dry zone. We constructed a species checklist for all herpetofauna of this area based on a short-term field survey, a comprehensive literature review, museum specimens, and observations made by field herpetologists. Based on 200 × 10 m belt transects, we surveyed herpetofauna both during day and night time, in 10 different types of habitats. The species checklist we compiled comprised 44 species of reptiles (including three nationally threatened, one globally threatened, and eight endemic species) and 15 species of amphibians (including one nationally threatened and three endemic species). Based on published literature, museum specimens, expert opinions, and current field survey, we documented 85 species of herpetofauna in this area. Of this entire list, we were unable to record the presence of 25 species through our field survey. Our field survey documented 18 species that were not previously reported from Jaffna Peninsula. Our study revealed that inland water bodies, cultivated lands, home gardens, and coastal beaches are of high impor- tance for native herpetofauna of Jaffna peninsula. Many human disturbances, such as habitat alterations, vengeful killing, consumption overexploitation, and road mortality are the key threats encountered by herpetofauna in Jaffna. -

Cop18 Prop. 23
Original language: English CoP18 Prop. 23 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal To include Calotes nigrilabris and Calotes pethiyagodai, in Appendix I in accordance with Res. Conf. 9.24 (Rev CoP17): I. Calotes nigrilabris meets Annex 1, criterion A (i), (v) as well as criterion B (i), (iii), (iv) and C (i), as a range-restricted species with small populations, which are highly fragmented; an observed decline in both habitat and number of individuals as well as their vulnerability to intrinsic and extrinsic factors are documented. II. Calotes pethiyagodai meets Annex 1, criterion A (i), (v) and criterion B (iii), (iv): it has small populations, is limited to an area of occupancy of less than 25 km2, is seriously affected by habitat loss and highly vulnerable to intrinsic and extrinsic factors. B. Proponent Sri Lanka*: C. Supporting statement 1. Taxonomy 1.1 Class: Reptilia 1.2 Order: Squamata 1.3 Family: Agamidae Calotes nigrilabris (Peters 1860) Calotes pethiyagodai (Amarasinghe et al. 2014) 1.4 Genus, species or subspecies, including author and year: 1.5 Scientific synonyms: C. nigrilabris: Calotes (Bronchocele) nigrilabris (Peters 1860) C. nigrilabris: Calotes rouxii BLYTH (Smith 1935) 1.6 Common names: English: * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. -

Sri Lanka: January 2015
Tropical Birding Trip Report Sri Lanka: January 2015 A Tropical Birding CUSTOM tour SRI LANKA: Ceylon Sojourn 9th- 23rd January 2015 Tour Leaders: Sam Woods & Chaminda Dilruk SRI LANKA JUNGLEFOWL is Sri Lanka’s colorful national bird, which was ranked among the top five birds of the tour by the group. All photos in this report were taken by Sam Woods. 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report Sri Lanka: January 2015 INTRODUCTION In many ways Sri Lanka covers it all; for the serious birder, even those with experience from elsewhere in the Indian subcontinent, it offers up a healthy batch of at least 32 endemic bird species (this list continues to grow, though, so could increase further yet); for those without any previous experience of the subcontinent it offers these but, being an island of limited diversity, not the overwhelming numbers of birds, which can be intimidating for the first timer; and for those with a natural history slant that extends beyond the avian, there is plentiful other wildlife besides, to keep all happy, such as endemic monkeys, strange reptiles only found on this teardrop-shaped island, and a bounty of butterflies, which feature day-in, day-out. It should also be made clear that while it appears like a chunk of India which has dropped of the main subcontinent, to frame it, as merely an extension of India, would be a grave injustice, as Sri Lanka feels, looks, and even tastes very different. There are some cultural quirks that make India itself, sometimes challenging to visit for the westerner. -

NHBSS 061 1G Hikida Fieldg
Book Review N$7+IST. BULL. S,$0 SOC. 61(1): 41–51, 2015 A Field Guide to the Reptiles of Thailand by Tanya Chan-ard, John W. K. Parr and Jarujin Nabhitabhata. Oxford University Press, New York, 2015. 344 pp. paper. ISBN: 9780199736492. 7KDLUHSWLOHVZHUHÀUVWH[WHQVLYHO\VWXGLHGE\WZRJUHDWKHUSHWRORJLVWV0DOFROP$UWKXU 6PLWKDQG(GZDUG+DUULVRQ7D\ORU7KHLUFRQWULEXWLRQVZHUHSXEOLVKHGDV6MITH (1931, 1935, 1943) and TAYLOR 5HFHQWO\RWKHUERRNVDERXWUHSWLOHVDQGDPSKLELDQV LQ7KDLODQGZHUHSXEOLVKHG HJ&HAN-ARD ET AL., 1999: COX ET AL DVZHOODVPDQ\ SDSHUV+RZHYHUWKHVHERRNVZHUHWD[RQRPLFVWXGLHVDQGQRWJXLGHVIRURUGLQDU\SHRSOH7ZR DGGLWLRQDOÀHOGJXLGHERRNVRQUHSWLOHVRUDPSKLELDQVDQGUHSWLOHVKDYHDOVREHHQSXEOLVKHG 0ANTHEY & GROSSMANN, 1997; DAS EXWWKHVHERRNVFRYHURQO\DSDUWRIWKHIDXQD The book under review is very well prepared and will help us know Thai reptiles better. 2QHRIWKHDXWKRUV-DUXMLQ1DEKLWDEKDWDZDVP\ROGIULHQGIRUPHUO\WKH'LUHFWRURI1DWXUDO +LVWRU\0XVHXPWKH1DWLRQDO6FLHQFH0XVHXP7KDLODQG+HZDVDQH[FHOOHQWQDWXUDOLVW DQGKDGH[WHQVLYHNQRZOHGJHDERXW7KDLDQLPDOVHVSHFLDOO\DPSKLELDQVDQGUHSWLOHV,Q ZHYLVLWHG.KDR6RL'DR:LOGOLIH6DQFWXDU\WRVXUYH\KHUSHWRIDXQD+HDGYLVHGXV WRGLJTXLFNO\DURXQGWKHUH:HFROOHFWHGIRXUVSHFLPHQVRIDibamusZKLFKZHGHVFULEHG DVDQHZVSHFLHVDibamus somsaki +ONDA ET AL 1RZ,DPYHU\JODGWRNQRZWKDW WKLVERRNZDVSXEOLVKHGE\KLPDQGKLVFROOHDJXHV8QIRUWXQDWHO\KHSDVVHGDZD\LQ +LVXQWLPHO\GHDWKPD\KDYHGHOD\HGWKHSXEOLFDWLRQRIWKLVERRN7KHERRNLQFOXGHVQHDUO\ DOOQDWLYHUHSWLOHV PRUHWKDQVSHFLHV LQ7KDLODQGDQGPRVWSLFWXUHVZHUHGUDZQZLWK H[FHOOHQWGHWDLO,WLVDYHU\JRRGÀHOGJXLGHIRULGHQWLÀFDWLRQRI7KDLUHSWLOHVIRUVWXGHQWV -

SACON News Vol 18 1
SACON News Vol. 18 (1) January – March 2021 Institutional Events Popular Articles New Director in charge, SACON 1 Studying a Wetland: Challenges 5 and Concerns Webinar on Wetlands 1 By Mythreyi Devarajan Webinar talk at Central 2 University of Kerala on the Beginnings to Big innings 9 occasion of National Science By Gourav Sonawane Day, 2021 Birds and invasives: An 11 Webinar talk at the 3 observation on Plum-headed International Symposium Parakeet Psittacula cyanocephala “Conservation of Life Below feeding on Parthenium Water” (COLIBA-2021) By Gayathri V, Thanikodi M organized by University of Kerala Talk at an online training 3 Researchers’ Corner— programme organized by Indian Art & Conservation Institute of Soil and water conservation Freezing a few moments with my 12 gregarious mates World Water Day 2021 4 By Priyanka Bansode Research Aptitude 4 An Illustration of Agamids and 13 Development Scheme (RADS) other lizards of Kerala digitally launched at Payyannur By Ashish A P college, Kerala Cover Page Photograph Credits Front: Indian Robin Feature Article Image ©Shantanu Nagpure ©Priyanka Bansode Back: Eurasian Collared Dove ©Deepak D. SACON News Vol 18(1), 2021 From the Director’s Desk It is my pleasure to invite the readers to this issue of SACON News. While we all hoped the New Year to have given us relief from Covid-19, unfortunately it has bounced back, perhaps with vengeance restricting our regular activities. Nevertheless, we got accustomed to an extent with many ‘new normals’, and continued with our tasks, nonetheless adhering to Covid-Appropriate norms. This issue of SACON News covers major activities of the institute and interesting articles from our research scholars. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Download Download
HAMADRYAD Vol. 27. No. 2. August, 2003 Date of issue: 31 August, 2003 ISSN 0972-205X CONTENTS T. -M. LEONG,L.L.GRISMER &MUMPUNI. Preliminary checklists of the herpetofauna of the Anambas and Natuna Islands (South China Sea) ..................................................165–174 T.-M. LEONG & C-F. LIM. The tadpole of Rana miopus Boulenger, 1918 from Peninsular Malaysia ...............175–178 N. D. RATHNAYAKE,N.D.HERATH,K.K.HEWAMATHES &S.JAYALATH. The thermal behaviour, diurnal activity pattern and body temperature of Varanus salvator in central Sri Lanka .........................179–184 B. TRIPATHY,B.PANDAV &R.C.PANIGRAHY. Hatching success and orientation in Lepidochelys olivacea (Eschscholtz, 1829) at Rushikulya Rookery, Orissa, India ......................................185–192 L. QUYET &T.ZIEGLER. First record of the Chinese crocodile lizard from outside of China: report on a population of Shinisaurus crocodilurus Ahl, 1930 from north-eastern Vietnam ..................193–199 O. S. G. PAUWELS,V.MAMONEKENE,P.DUMONT,W.R.BRANCH,M.BURGER &S.LAVOUÉ. Diet records for Crocodylus cataphractus (Reptilia: Crocodylidae) at Lake Divangui, Ogooué-Maritime Province, south-western Gabon......................................................200–204 A. M. BAUER. On the status of the name Oligodon taeniolatus (Jerdon, 1853) and its long-ignored senior synonym and secondary homonym, Oligodon taeniolatus (Daudin, 1803) ........................205–213 W. P. MCCORD,O.S.G.PAUWELS,R.BOUR,F.CHÉROT,J.IVERSON,P.C.H.PRITCHARD,K.THIRAKHUPT, W. KITIMASAK &T.BUNDHITWONGRUT. Chitra burmanica sensu Jaruthanin, 2002 (Testudines: Trionychidae): an unavailable name ............................................................214–216 V. GIRI,A.M.BAUER &N.CHATURVEDI. Notes on the distribution, natural history and variation of Hemidactylus giganteus Stoliczka, 1871 ................................................217–221 V. WALLACH. -

Zoology ABSTRACT
Research Paper Volume : 3 | Issue : 9 | September 2014 • ISSN No 2277 - 8179 New record of Roux’s Forest Lizard Calotes Zoology rouxii (Duméril & Bibron, 1837) (Reptilia: KEYWORDS : Calotes rouxii, range exten- Squamata: Agamidae) from Sandur and sion, distribution update, Karnataka Gulbarga, Karnataka, India with a note on its known distribution Biodiversity Research and Conservation Society, 303 Orchid, Sri Sai Nagar Colony, Aditya Srinivasulu Kanajiguda, Secunderabad, Telangana 500 015, India. Natural History Museum and Wildlife Biology and Taxonomy Lab, Department of Zoology, * C. Srinivasulu University College of Science, Osmania University, Hyderabad, Telangana 500 007, India. *Corresponding Author ABSTRACT Roux’s Forest Lizard Calotes rouxii (Duméril & Bibron, 1837) is chiefly a forest-dwelling draconine agamid that is widely distributed in the Western Ghats and occasionally reported from the Eastern Ghats and other localities in the central peninsular India. We report the presence of this species for the first time from Sandur forests in Bellary district, Karnataka based on a voucher specimen and from Gulbarga township based on sighting record. A detailed distribution map showing localities from where the species is known is also provided. The genus Calotes Daudin, 1802, belonging to the draconian fam- ized by the presence of a dewlap in males, two slender spines on ily Agamidae (Reptilia) is native to South Asia, South-East Asia either side of the head and a dark groove before the shoulder. and Southern China. It is represented -
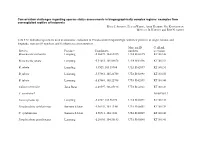
Conservation Challenges Regarding Species Status Assessments in Biogeographically Complex Regions: Examples from Overexploited Reptiles of Indonesia KYLE J
Conservation challenges regarding species status assessments in biogeographically complex regions: examples from overexploited reptiles of Indonesia KYLE J. SHANEY, ELIJAH WOSTL, AMIR HAMIDY, NIA KURNIAWAN MICHAEL B. HARVEY and ERIC N. SMITH TABLE S1 Individual specimens used in taxonomic evaluation of Pseudocalotes tympanistriga, with their province of origin, latitude and longitude, museum ID numbers, and GenBank accession numbers. Museum ID GenBank Species Province Coordinates numbers accession Bronchocela cristatella Lampung -5.36079, 104.63215 UTA R 62895 KT180148 Bronchocela jubata Lampung -5.54653, 105.04678 UTA R 62896 KT180152 B. jubata Lampung -5.5525, 105.18384 UTA R 62897 KT180151 B. jubata Lampung -5.57861, 105.22708 UTA R 62898 KT180150 B. jubata Lampung -5.57861, 105.22708 UTA R 62899 KT180146 Calotes versicolor Jawa Barat -6.49597, 106.85198 UTA R 62861 KT180149 C. versicolor* NC009683.1 Gonocephalus sp. Lampung -5.2787, 104.56198 UTA R 60571 KT180144 Pseudocalotes cybelidermus Sumatra Selatan -4.90149, 104.13401 UTA R 60551 KT180139 P. cybelidermus Sumatra Selatan -4.90711, 104.1348 UTA R 60549 KT180140 Pseudocalotes guttalineatus Lampung -5.28105, 104.56183 UTA R 60540 KT180141 P. guttalineatus Sumatra Selatan -4.90681, 104.13457 UTA R 60501 KT180142 Pseudocalotes rhammanotus Lampung -4.9394, 103.85292 MZB 10804 KT180147 Pseudocalotes species 4 Sumatra Barat -2.04294, 101.31129 MZB 13295 KT211019 Pseudocalotes tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60544 KT180143 P. tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60547 KT180145 Pogona vitticeps* AB166795.1 *Entry to GenBank by previous authors TABLE S2 Reptile species currently believed to occur Java and Sumatra, Indonesia, with IUCN Red List status, and certainty of occurrence. -

Myanmar National Red List Training and Assessment Workshop Forest Research Institute, Yezin, Naypyitaw 23-27 July 2018
Myanmar National Red List Training and Assessment Workshop Forest Research Institute, Yezin, Naypyitaw 23-27 July 2018 1 Background The Myanmar National Biodiversity Strategy and Action Plan 2015-2020 includes Target 12.3 “By 2020, a National Red List of selected taxa has been produced” and Actions 12.3.1 “Conduct Red List assessments for key taxa, with a particular focus on endemic species” and 12.3.2 “Hold training workshops to build capacity on application of the Red List categories and criteria”. In July 2017, in partnership with the Ministry of Natural Resources and Environmental Conservation’s (MONREC’s) Nature and Wildlife Conservation Division (NWCD), WCS Myanmar, and WWF Myanmar, IUCN organized a 5-day Red List Assessor training workshop at the Forest Research Institute (FRI), Yezin, near Naypyitaw. 30 participants attended, representing organizations including the Myanmar Forest Department, WCS, WWF, FFI, Instituto Oikos, Myanmar Floriculturalist Association, Taunggyi University, Yangon University, Mandalay University, and Myitkyinar University. In addition to building national capacity in use of the Red List Categories and Criteria, this event was intended to initiate the process of developing a Myanmar National Red List. It was followed by the formal establishment of five National Red List Working Groups – focusing on mammals, birds, reptiles and amphibians, plants, and aquatic species, approved by the MONREC Minister. In July 2018, IUCN continued to support the development of a Myanmar National Red List by facilitating a second Red List workshop at FRI, Naypyitaw, again in partnership with NWCD, WCS Myanmar, and WWF Myanmar. This event consisted of three days of Red List Assessor training for all members of the Myanmar National Red List Working Groups (23-25 July), followed by two days of conducting national-level assessments of reptile species with the Myanmar National Red List Reptiles and Amphibians Working Group (26-27 July). -

On the Occurrences of Japalura Kumaonensis and Japalura Tricarinata (Reptilia: Sauria: Draconinae) in China
Herpetologica, 74(2), 2018, 181–190 Ó 2018 by The Herpetologists’ League, Inc. On the Occurrences of Japalura kumaonensis and Japalura tricarinata (Reptilia: Sauria: Draconinae) in China 1,2 3,4 5 6 7 3,4 1 KAI WANG ,KE JIANG ,V.DEEPAK ,DAS ABHIJIT ,MIAN HOU ,JING CHE , AND CAMERON D. SILER 1 Sam Noble Oklahoma Museum of Natural History and Department of Biology, University of Oklahoma, Norman, OK 73072, USA 3 Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan 650223, China 4 Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Yunnan 666303, China 5 Center for Ecological Sciences, Indian Institute of Science, Bangalore, Karnataka 560012, India 6 Wildlife Institute of India, Chandrabani, Dehradun 248002, India 7 Academy of Continuing Education, Sichuan Normal University, Chengdu, Sichuan 610068, China ABSTRACT: Although the recognized distribution of Japalura kumaonensis is restricted largely to western Himalaya, a single, isolated outlier population was reported in eastern Himalaya at the China-Nepal border in southeastern Tibet, China in Zhangmu, Nyalam County. Interestingly, subsequent studies have recognized another morphologically similar species, J. tricarinata, from the same locality in Tibet based on photographic evidence only. Despite these reports, no studies have examined the referred specimens for either record to confirm their taxonomic identifications with robust comparisons to congener species. Here, we examine the referred specimen of the record of J. kumaonensis from southeastern Tibet, China; recently collected specimens from the same locality in southeastern Tibet; type specimens; and topotypic specimens of both J. kumaonensis and J. tricarinata, to clarify the taxonomic identity of the focal population from southeastern Tibet, China. -

The Ecology of Lizard Reproductive Output
Global Ecology and Biogeography, (Global Ecol. Biogeogr.) (2011) ••, ••–•• RESEARCH The ecology of lizard reproductive PAPER outputgeb_700 1..11 Shai Meiri1*, James H. Brown2 and Richard M. Sibly3 1Department of Zoology, Tel Aviv University, ABSTRACT 69978 Tel Aviv, Israel, 2Department of Biology, Aim We provide a new quantitative analysis of lizard reproductive ecology. Com- University of New Mexico, Albuquerque, NM 87131, USA and Santa Fe Institute, 1399 Hyde parative studies of lizard reproduction to date have usually considered life-history Park Road, Santa Fe, NM 87501, USA, 3School components separately. Instead, we examine the rate of production (productivity of Biological Sciences, University of Reading, hereafter) calculated as the total mass of offspring produced in a year. We test ReadingRG6 6AS, UK whether productivity is influenced by proxies of adult mortality rates such as insularity and fossorial habits, by measures of temperature such as environmental and body temperatures, mode of reproduction and activity times, and by environ- mental productivity and diet. We further examine whether low productivity is linked to high extinction risk. Location World-wide. Methods We assembled a database containing 551 lizard species, their phyloge- netic relationships and multiple life history and ecological variables from the lit- erature. We use phylogenetically informed statistical models to estimate the factors related to lizard productivity. Results Some, but not all, predictions of metabolic and life-history theories are supported. When analysed separately, clutch size, relative clutch mass and brood frequency are poorly correlated with body mass, but their product – productivity – is well correlated with mass. The allometry of productivity scales similarly to metabolic rate, suggesting that a constant fraction of assimilated energy is allocated to production irrespective of body size.