Isolation and Identification of Compounds Conferring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Botanicals in Postmenopausal Osteoporosis
nutrients Review Botanicals in Postmenopausal Osteoporosis Wojciech Słupski, Paulina Jawie ´nand Beata Nowak * Department of Pharmacology, Wroclaw Medical University, ul. J. Mikulicza-Radeckiego 2, 50-345 Wrocław, Poland; [email protected] (W.S.); [email protected] (P.J.) * Correspondence: [email protected]; Tel.: +48-607-924-471 Abstract: Osteoporosis is a systemic bone disease characterized by reduced bone mass and the deterioration of bone microarchitecture leading to bone fragility and an increased risk of fractures. Conventional anti-osteoporotic pharmaceutics are effective in the treatment and prophylaxis of osteoporosis, however they are associated with various side effects that push many women into seeking botanicals as an alternative therapy. Traditional folk medicine is a rich source of bioactive compounds waiting for discovery and investigation that might be used in those patients, and therefore botanicals have recently received increasing attention. The aim of this review of literature is to present the comprehensive information about plant-derived compounds that might be used to maintain bone health in perimenopausal and postmenopausal females. Keywords: osteoporosis; menopause; botanicals; herbs 1. Introduction Women’s health and quality of life is modulated and affected strongly by hormone status. An oestrogen level that changes dramatically throughout life determines the Citation: Słupski, W.; Jawie´n,P.; development of women’s age-associated diseases. Age-associated hormonal imbalance Nowak, B. Botanicals in and oestrogen deficiency are involved in the pathogenesis of various diseases, e.g., obesity, Postmenopausal Osteoporosis. autoimmune disease and osteoporosis. Many female patients look for natural biological Nutrients 2021, 13, 1609. https:// products deeply rooted in folk medicine as an alternative to conventional pharmaceutics doi.org/10.3390/nu13051609 used as the prophylaxis of perimenopausal health disturbances. -

Effects of Phytoestrogens in the Treatment of Postmenopausal
Effects of phytoestrogens in the treatment of postmenopausal depressive disorders: A systematic review and meta-analysis Li Jieyun1, Huijuan LI1, Yan Peijing1, Jingwen Li1, Jie Qiu1, Jiani Han1, and Kehu Yang2 1Affiliation not available 2Evidence Based Medicine Center July 20, 2020 Abstract Background:Menopause-related symptoms are now a major public health concern and depression is one of the most common and specific symptoms of post-menopause. Objectives: The purpose of this study was to assess the effectiveness of different kinds of phytoestrogens in the remission of depression for postmenopausal women. Methods: A comprehensive search for relevant studies published between January 1, 1951 and June 30, 2020 was conducted in PubMed, the Cochrane Library, Chinese Biomedical Literature Database, Web of Science and EMBASE. Endnote X9 was used for screening articles, the Stata12.0 and Review Manager 5.3 for analyzing data. Results: Phytoestrogens had a have a significant positive effect on depressive symptoms for climacteric women compared to the placebo (SMD=-0.51; 95% [CI]=-0.74 to -0.29; I2=72.4%; P<0.05). The effectiveness in isoflavones (SMD=-0.64; 95% [CI]=-0.97 to -0.32; I2=56.2%, P>0.05) is slightly better than non-isoflavones (SMD=-0.5; 95%[CI]=-93 to -0.07; I2=72.8%, P<0.05). The total intake of phytoestrogens in the high dose group was the most effective in alleviating the symptoms of postmenopausal depression (SMD=-0.69; 95%CI=-1.09 to -0.35, P<0.05). Postmenopausal women in Europe had the best improvement in efficacy after taking phytoestrogens (SMD=-0.72, 95% CI=-1.09 to -0.35, I2=65.5%, P<0.05). -

Consolidated List of Article 13 Health Claims List of References Received by EFSA
Unit on Dietetic Products, Nutrition and Allergies Parma, 5 April 2011 Consolidated list of Article 13 health claims List of references received by EFSA Part 3 IDs 2001 – 3000 (This document contains the list of references for claims which the Commission has asked EFSA to prioritise in the evaluation.) BACKGROUND In accordance with Article 13 of Regulation (EC) No 1924/20061 Member States had provided the European Commission with lists of claims accompanied by the conditions applying to them and by references to the relevant scientific justification by 31 January 2008. EFSA has received from the European Commission nine Access databases with a consolidated list of 4,185 main health claim entries with around 10,000 similar health claims. The similar health claims were accompanied by the conditions of use and scientific references. The nine Access databases were sent in three batches - in July 2008, in November 2008 and in December 2008. Subsequently, EFSA combined the databases into one master database and re-allocated upon request of the Commission and Member States similar health claims which had been accidentally placed under a wrong main health claim entry (misplaced claims). During this process some Member States also identified a number of similar health claims which still needed to be submitted to EFSA (―missing claims‖). These similar claims were also added to the database. In March 2010, the European Commission forwarded to EFSA an addendum to the consolidated list containing an additional 452 main entry claims which have been added to the updated final database which was published on the EFSA website in May 2010 (containing 4,637 main entry claims). -

Dietary Phyto-Oestrogens and Bone Health
Proceedings of the Nutrition Society (2003), 62, 877–887 DOI:10.1079/PNS2003309 © The Author 2003 CABPNS88711© Nutrition309OptimumInternationalPNSProceedings Society nutrition 2003 for osteoporosis of Nutrition preventionF. Society (2003)0029-6651© Branca877 Nutrition Society 2003 624 Dietary phyto-oestrogens and bone health Francesco Branca Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione (INRAN), Via Ardeatina 546, 00178 Rome, Italy Professor Francesco Branca, fax +39 06 51494 550, email [email protected] The use of dietary phyto-oestrogens as a possible option for the prevention of osteoporosis has raised considerable interest because of the increased concern about the risks associated with the use of hormone-replacement therapy. However, the evidence in support of a bone-sparing effect in post-menopausal women is still not sufficiently convincing. Most studies have been performed on soyabean isoflavones (genistein and daidzein), either in the purified form or as a soyabean- based product or extract. In vitro studies using primary cell cultures or stabilised cell lines indicate that treatment with genistein may lead to a reduction in bone resorption, but effects on bone formation have also been shown. Investigations using animal models have provided convincing evidence of major improvements in bone mass or bone turnover following soyabean feeding. Cross-sectional observations in South-East Asian populations with moderately high intakes of soyabean isoflavones (50 mg/d) have shown that women in the high quartile of intake have higher bone mineral density (BMD) and reduced bone turnover, an effect that has not been shown in populations with low average intakes. Human trials have given an indication of a possible effect on lumbar spine BMD, although they have been either short term (< 6 months) or methodologically weak. -

Phytoestrogens for Menopausal Vasomotor Symptoms (Review)
Phytoestrogens for menopausal vasomotor symptoms (Review) Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2013, Issue 12 http://www.thecochranelibrary.com Phytoestrogens for menopausal vasomotor symptoms (Review) Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 3 OBJECTIVES ..................................... 4 METHODS ...................................... 5 Figure1. ..................................... 7 Figure2. ..................................... 8 Figure3. ..................................... 9 RESULTS....................................... 11 Figure4. ..................................... 16 DISCUSSION ..................................... 18 AUTHORS’CONCLUSIONS . 22 ACKNOWLEDGEMENTS . 22 REFERENCES ..................................... 23 CHARACTERISTICSOFSTUDIES . 32 DATAANDANALYSES. 99 Analysis 1.1. Comparison 1 Promensil versus placebo, Outcome 1 Incidence of hot flushes (number/d). 100 Analysis 1.2. Comparison 1 Promensil versus placebo, Outcome 2 Change in frequency of hot flushes (% reduction). 101 Analysis 1.3. Comparison 1 Promensil versus placebo, Outcome 3 Improvement in hot flush severity rate. 101 Analysis 1.4. Comparison 1 Promensil versus placebo, -

Soy Isoflavone Intake Inhibits Bone Resorption and Stimulates Bone Formation in Menopausal Women: Meta-Analysis of Randomized Controlled Trials
European Journal of Clinical Nutrition (2008) 62, 155–161 & 2008 Nature Publishing Group All rights reserved 0954-3007/08 $30.00 www.nature.com/ejcn ORIGINAL ARTICLE Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials D-F Ma1,2, L-Q Qin3, P-Y Wang1 and R Katoh2 1Department of Social Medicine and Health Education, School of Public Health, Peking University, Beijing, China; 2Department of Human Pathology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi, Japan and 3Department of Nutrition and Food Hygiene, School of Radiation Medicine and Public Health, Soochow University, Suzhou, China Objective: To clarify the effects of isoflavone intake on bone resorption and bone formation. Methods: We identified randomized controlled trials related to urinary deoxypyridinoline (Dpyr, a bone resorption marker) and serum bone-specific alkaline phosphatase (BAP, a bone formation marker) listed on MEDLINE (January 1966–April 2006), the Cochrane Controlled Trials Register, EMBASE (1985–January 2006), Science Citation Index and PUBMED (updated till April 2006). Results: Nine studies with a total of 432 subjects were selected for meta-analysis. The urinary Dpyr concentration in subjects who consumed isoflavones decreased significantly by À2.08 nmol/mmol (95% confidence interval (CI): À3.82 to À0.34 nmol/ mmol) in comparison with that in subjects who did not consume isoflavones. Isoflavone intake vs placebo intake significantly increased serum BAP by 1.48 mg/l (95% CI: 0.22–2.75 mg/l). Decreases in the urinary Dpyr concentration with isoflavone intake of o90 mg/day and with treatment lasting less than 12 weeks were À2.34 nmol/mmol (95% CI: À4.46 to À0.22 nmol/mmol) and À2.03 nmol/mmol (95% CI: À3.20 to À0.85 nmol/mmol), respectively. -

Downloaded for Personal Use Only
Paul Cos Tess De Bruyne Sandra Apers Dirk Vanden Berghe Luc Pieters Arnold J.Vlietinck Phytoestrogens: Recent Developments Review Abstract daidzein, coumestrol and equol, several prenylated flavonoids, especially 8-prenylnaringenin, and the stilbene resveratrol. Fur- Phytoestrogens are polyphenolic non-steroidal plant compounds thermore, their non-hormonal effects will be discussed briefly. with estrogen-like biological activity. Based on their chemical Finally, the latest developments on the potential protective prop- structure, phytoestrogens can be classified into four main erties of phytoestrogens and phytoestrogen-containing foods groups, i.e., isoflavonoids, flavonoids, stilbenes, and lignans. For against hormone-dependent breast and prostate cancers and each group, the chemistry, dietary sources and biotransforma- cardiovascular diseases, and as estrogen replacement therapy tion of the most interesting compounds will be discussed. Since for postmenopausal women will be discussed. phytoestrogens are structurally very similar to the estrogen 17b- estradiol, they may exhibit selective estrogen receptor modulat- Key words ing activities. Therefore, special attention will be given to the Phytoestrogens ´ isoflavones ´ resveratrol ´ lignans ´ hop ´ estrogen hormonal effects of various isoflavonoids, including genistein, receptor Introduction concerns have been raised about the potential dangers of con- 589 suming high levels of these compounds [8]. Consequently, phy- Phytoestrogens are polyphenolic non-steroidal plant compounds toestrogens are currently under active investigation for their with estrogen-like biological activity. The estrogenic properties role on human health. of certain plants have been recognized for more than fifty years. In the mid-1940s, an infertility syndrome in sheep had been at- This review will discuss the recent literature on phytoestrogens, tributed to the ingestion of clover containing high levels of the focusing on their biological effects and biotransformation, as isoflavones formononetin (1) and biochanin A (2) [1], [2]. -

Original Article Alleviative Effects of Yukmijihwang-Tang on Cholesterol Related Disease in a Postmenopausal Rat Model and Lipid Accumulation in Hepg2 Cells
Int J Clin Exp Med 2016;9(6):10093-10108 www.ijcem.com /ISSN:1940-5901/IJCEM0022373 Original Article Alleviative effects of Yukmijihwang-tang on cholesterol related disease in a postmenopausal rat model and lipid accumulation in HepG2 cells Hiroe Go1, Jin Ah Ryuk1, Hye Won Lee1, Ki-Jung Kil2, Byoung Seob Ko1 1Korean Medicine Convergence Research Division, Korea Institute of Oriental Medicine, Republic of Korea; 2Col- lege of Oriental Medicine, Joongbu University, Republic of Korea Received December 21, 2015; Accepted April 3, 2016; Epub June 15, 2016; Published June 30, 2016 Abstract: Yukmijihwang-tang (YMJT), known as Liu-wei-di-huang-tang in Chinese and Lokumijio-to in Japanese, has been used as a traditional herbal formula for the treatment of various diseases. In Korean traditional medicine, YMJT is a well-known herbal prescription used to relieve Yin-Deficiency. In this study, we examined the effects of YMJT on Nonalcoholic steatohepatitis (NASH) and on diseases associated with cholesterol in a rat model of post- menopausal hyperlipidemia and the methyl-β-cyclodextrin (MβCD)-induced hepatic steatosis model in HepG2 cells. Twenty-five rats were ovariectomized (OVX), and five rats were sham-operated (Sham). Then, the OVX rats were randomly assigned to three groups (n = 5): OVX-Con, OVX with simvastatin, and OVX with YMJT (50, 150, 450 mg/ kg) for 8 weeks. NASH and a number of targets associated with cholesterol were examined to confirm the effects of YMJT. Oil Red O staining and intracellular cholesterol analyses were used to quantify cellular cholesterol levels. The levels of phosphorylated AMP-activated protein kinases (AMPK) and the products of genes involved in cholesterol synthesis were measured via Western blotting. -

Phytoestrogens: a Review of the Present State of Research
PHYTOTHERAPY RESEARCH Phytother. Res. 17, 845–869 (2003) Published online in Wiley InterScience (www.interscience.wiley.com).PHYTOESTROGENS DOI: 10.1002/ptr.1364 845 REVIEW ARTICLE Phytoestrogens: a Review of the Present State of Research Andreana L. Ososki1,2,3 and Edward J. Kennelly1,2* 1Biological Sciences, Lehman College, City University of New York, 250 Bedford Park Blvd West, Bronx, NY 10468, USA 2City University of New York Graduate Center, 365 Fifth Avenue, New York, NY 10016, USA 3Institute of Economic Botany, New York Botanical Garden, 200 Southern Blvd, Bronx, NY 10458, USA Phytoestrogens are a diverse group of plant-derived compounds that structurally or functionally mimic mammalian estrogens and show potential benefits for human health. The number of articles published on phytoestrogens has risen dramatically in the past couple decades. Further research continues to demonstrate the biological complexity of phytoestrogens, which belong to several different chemical classes and act through diverse mechanisms. This paper discusses the classification of phytoestrogens, methods of identification, their proposed mechanisms of action and botanical sources for phytoestrogens. The effects of phytoestrogens on breast and prostate cancers, cardiovascular disease, menopausal symptoms and osteoporosis will also be examined including research on benefits and risks. Copyright © 2003 John Wiley & Sons, Ltd. Keywords: botanicals; coumestans; isoflavones; lignans; phytoestrogens. further confirmed by the recent suspension of the INTRODUCTION Women’s -

Pleiotropic Effects of Isoflavones in Inflammation and Chronic
International Journal of Molecular Sciences Review Pleiotropic Effects of Isoflavones in Inflammation and Chronic Degenerative Diseases Jurga Bernatoniene 1,2,* , Jurga Andreja Kazlauskaite 2 and Dalia Marija Kopustinskiene 2 1 Department of Drug Technology and Social Pharmacy, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania 2 Institute of Pharmaceutical Technologies, Faculty of Pharmacy, Medical Academy, Lithuanian University of Health Sciences, Sukileliu pr. 13, LT-50161 Kaunas, Lithuania; [email protected] (J.A.K.); [email protected] (D.M.K.) * Correspondence: [email protected] Abstract: Isoflavones are phytoestrogens of plant origin, mostly found in the members of the Fabaceae family, that exert beneficial effects in various degenerative disorders. Having high similarity to 17-b- estradiol, isoflavones can bind estrogen receptors, scavenge reactive oxygen species, activate various cellular signal transduction pathways and modulate growth and transcription factors, activities of enzymes, cytokines, and genes regulating cell proliferation and apoptosis. Due to their pleiotropic activities isoflavones might be considered as a natural alternative for the treatment of estrogen decrease-related conditions during menopause. This review will focus on the effects of isoflavones on inflammation and chronic degenerative diseases including cancer, metabolic, cardiovascular, neurodegenerative diseases, rheumatoid arthritis and adverse postmenopausal symptoms. Keywords: isoflavones; genistein; daidzein; inflammation; cancer; degenerative diseases Citation: Bernatoniene, J.; Kazlauskaite, J.A.; Kopustinskiene, D.M. Pleiotropic Effects of Isoflavones in Inflammation and Chronic Degenerative Diseases. Int. J. Mol. Sci. 1. Introduction 2021, 22, 5656. https://doi.org/ Isoflavones are polyphenolic plant-derived compounds acting as phytoestrogens due 10.3390/ijms22115656 to their structural similarity to 17-b-estradiol [1]. -
Pharmaceutical Prospects of Phytoestrogens
Endocrine Journal 2006, 53 (1), 7–20 REVIEW Pharmaceutical Prospects of Phytoestrogens TAKESHI USUI Division of Endocrinology, Clinical Research Center, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan Abstract. Interest in the physiologic and pharmacologic role of bioactive compounds present in plants has increased dramatically over the last decade. Of particular interest in relation to human health are the classes of compounds known as the phytoestrogens, which embody several groups of non-steroidal estrogens, including isoflavones and lignans that are widely distributed within nature. The impact of dietary phytoestrogens on normal biologic processes was first recognized in sheep. Observations of sheep grazing on fields rich in clover and cheetahs fed high soy diets in zoos suggested that flavonoids and related phytochemicals can affect mammalian health. Endogenous estrogens have an important role not only in the hypothalamic-pituitary-gonadal axis, but also in various non-gonadal systems, such as cardiovascular systems, bone, and central nervous systems, and lipid metabolism. There have been several clinical studies of hormone replacement therapy (HRT) in post-menopausal women to examine whether HRT has beneficial effects on the cardiovascular system, bone fractures, lipid metabolism, and Alzheimer’s disease. In addition, estrogen contributes to the development of some estrogen-dependent cancers, such as breast cancer and prostate cancer and the number of patients with these cancers is increasing in developed countries. Although recent mega-studies showed negative results for classical HRT in the prevention of some of these diseases, the molecules that interact with estrogen receptors are candidate drugs for various diseases, including hormone-dependent cancers. -
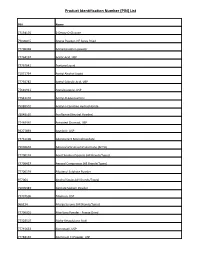
Product Identification Number (PIN) List
Product Identification Number (PIN) List PIN Name 77154176 2-Deoxy-D-Glucose 79000015 Acacia Powder, NF Spray Dried 77700469 Acetaminophen powder 77764197 Acetic Acid, USP 77767641 Acetone Liquid 71071734 Acetyl Alcohol Liquid 77750782 Acetyl Salicylic Acid, USP 77616911 Acetylcysteine, USP 77512176 Acetyl-D-Glucosamine 75080502 Acetyl-L-Carnitine Hydrochloride 78048520 Acriflavine (Neutral Powder) 77440440 Activated Charcoal, USP 59277893 Acyclovir, USP 77761198 Adenosine-5 Monophosphate 79002602 Adrenocorticotrophic Hormone (ACTH) 77700174 AeroChamber/Spacers (All Brands/Types) 77700427 Aerosol Compressor (All Brands/Types) 77700529 Albuterol Sulphate Powder 977064 Alcohol Swabs (All Brands/Types) 79005383 Alginate Sodium Powder 77797596 Allantoin, USP 966134 Allergy Serums (All Brands/Types) 77700001 Aloe Vera Powder - Freeze Dried 77328507 Alpha-Ketoglutaric Acid 77745653 Alprostadil, USP 77784136 Aluminum Cl Powder, USP Product Identification Number (PIN) List 77446700 Aluminum Cl Powder, USP (anhydrous) 901997 Amidon Powder 77700544 Aminobenzoic acid 77760322 Aminocaproic Acid, USP 77700521 Aminophylline Powder 77549188 Amitriptyline Hydrochloride, USP 78000735 Ammonium Carbonate, NF 71397893 Amphotericin B, USP (Oral Grade) 77700036 Anhydrous Eucerin 71143380 Anthralin 92099969 Antibiotic adherence (follow-up) 92099968 Antibiotic adherence (Initial) 41372207 Apomorphine Hydrochloride,USP 77700472 Applicator (All Brands/Types/Sizes) Anurex/Silver Nitrate 71119342 Arginine Hydrochloride,USP 77774793 Arginine, USP 77750817 Ascorbic