Federal Register/Vol. 85, No. 244/Friday
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
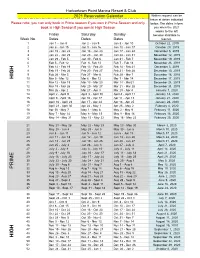
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Updated Calendar: November 23 - December 18
Updated calendar: November 23 - December 18 November 23 - 24 ● Nov-23: A-K in-person, L-Z remote ● Nov-24: L-Z in-person, A-K remote November 25 - November 29 ● No school - Thanksgiving Break November 30 - December 4 ● Nov-30: Teacher in-service; no remote learning for students. ● Dec-1 to Dec-4: Remote learning for all students (A-Z). The Boylan bell schedule will be used when teachers hold Google Meets. December 7 - 11 ● Remote learning for all students (A-Z). The Boylan bell schedule will be used when teachers hold Google Meets. ● Review positivity rate of community and region to determine plans for 12/14 - 12/18. December 14 - 18 ● If data indicates it is safe to return to school, then we will use alternating cohorts. ○ 12/14: A-K in-person, L-Z remote ○ 12/15: L-Z in-person, A-K remote ○ 12/16: All students remote ○ 12/17: A-K in-person, L-Z remote ○ 12/18: L-Z in-person, A-K remote ● If data indicates it’s unsafe to return or staffing issues don’t allow for in-person learning, then we will continue with remote learning. December 19 - January 3 ● No school - Christmas Break January 4 - 15 ● Jan-4: Teacher in-service; no remote learning for students. ● Jan-5 to Jan-15: Remote learning for all students (A-Z). The Boylan bell schedule will be used when teachers hold Google Meets. January 18 ● No school - Martin Luther King Jr. Day January 19 - March 12 ● Continue to use alternating cohorts. Specific calendar dates will be released at a later time. -

Cuesta Student Planning Calendar
SUMMER SESSION 2021: June 14 – July 23 WINTER BREAK: December 18 - January 17 March 26……….....……...Class Finder available online 2021-2022 December 23–24, 27–30..…..................Board Holidays Beginning April 1………………………….…Apply online STUDENT December 31.……New Year’s Day Holiday (observed) April 19….......……………Priority myCuesta reg begins PLANNING CALENDAR January 12–14, 2022.…..……...……………..Flex Days April 22.………...New/returning/transfer student priority January 17, 2022……….Martin Luther King, Jr Holiday April 27..…............Enrichment/Dual Enrollment priority June 2021 December 2021 May 1...…..…International student application deadline U M T W R F S U M T W R F S SPRING SEMESTER 2022: January 18 – May 20 Day Prior to 1st Class Meeting……..Drop for full refund 1 2 3 4 5 1 2 3 4 6 7 8 9 10 11 12 5 6 7 8 9 10 11 Day Prior to Census Date……..Late add with add code Beginning October 1, 2021…...…......…….Apply online 13 14 15 16 17 18 19 12 13 14 15 16 17 18 October 29..………..…….Class Finder available online July 5 (observed).........……Independence Day Holiday 20 21 22 23 24 25 26 19 20 21 22 23 24 25 27 28 29 30 26 27 28 29 30 31 November 9……..………..Priority myCuesta reg begins July 2021 January 2022 November 22…. New/returning/transfer student priority U M T W R F S U M T W R F S FALL SEMESTER 2021: August 16 – December 17 1 2 3 1 December 1..………International student application deadline 4 5 6 7 8 9 10 2 3 4 5 6 7 8 Beginning October 1, 2020....………..…….Apply online December 3…..…Enrichment/Dual Enrollment student priority 11 12 13 14 15 -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
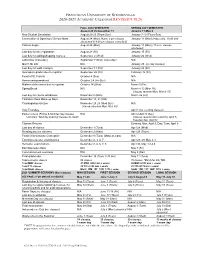
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

2021 Subdivision Calendar
Planning Department Development Review – Major Subdivision Applications First Plat Review 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Deadline for Staff Planning Board BOC Meeting Planning Department Review Deadline Postponement Meeting Date* Date** December 18, 2020 December 31 January 13 January 22 February 2 March 15 January 15 January 29 February 10 February 19 March 2 April 19 February 19 March 5 March 17 March 26 April 6 May 17 March 19 April 2 April 14 April 23 May 4 June 21 April 16 April 30 May 12 May 21 June 1 July 19 May 21 June 4 June 16 June 25 July 6 August 16 June 18 July 2 July 14 July 23 August 3 September 20 July 23 August 6 August 18 August 27 September 7 October 18 August 20 September 3 September 15 September 24 October 5 November 15 September 17 October 1 October 13 October 22 November 2 December 20 October 22 November 5 November 17 November 26 December 7 ▪ January 18, 2022 November 19 December 3 December 15 ▪ December 22 ▪ January 4, ▪ February 21, 2022 2022 ▪ ▪ ▪ December 17 December 31, January 12, ▪ January 21, ▪ February 1, March 21, 2021 2022 2022 2022 2022 * Planning Board Has Two (2) Meetings to Make Recommendation ** Board of Commissioners Has Up to Four (4) Meetings to Make Decision ▪ 2021 Dates are Tentative – Meeting Dates Will Be Set in December 2021 With Adoption of 2022 Meeting Calendars Construction/Final Plat Review – Administrative* 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Planning Review Deadline Department For TRC December 23, 2020 January 6 January 13 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

195 Revised 12/20
2020 — 2021 NORMAN PUBLIC SCHOOLS 2020 — 2021 LENGTH OF CONTRACT Beginning Date: July 24, 2020 NUMBER OF NON-DUTY DAYS NUMBER OF VACATION DAYS HOLIDAYS X 195 Ending Date: June 3, 2021 66 0 0 STAFF - NON-DUTY DAYS OPAT Parent Educator July 1-3, 6-10, 13-17, 20-23 Nov 23-27 Mar 15-19 Fine Arts Secretary (Sec #2) Aug Dec 21-25, 28-31 Apr Site Athletic/Activities Director Sept 7 Jan 1, 4, 18 May 31 Oct 8-9 Feb June 4, 7-11, 14-18, 21-25, 28-30 July 2020 August 2020 September 2020 Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat 1 2 3X 4 1 1 2 3 4 5 5 6 7 8 9 10 11 2 3 4 5 6 7 8 6 7X 8 9 10 11 12 SICK LEAVE 12 13 14 15 16 17 18 9 10� 11� 12� 13� 14� 15 13 14 15 16 17 18 19 19 20 21 22 23 24 25 16 17� 18� 19� 20� 21� 22 20 21 22 23 24 25 26 10 26 27 28 29 30 31 23 24❤ 25 26 27 28 29 27 28 29 30 � 30 31 NUMBER OF DAYS October 2020 November 2020 December 2020 WORKED PER MONTH Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat 1 2 3 1 2 3 4 5 6 7 1 2 3 4 5 July 6 4 5 6 7 8 9 10 8 9 10 11 12 13 14 6 7 8 9 10 11 12 Aug 21 11 12 13 14 15 16 17 15 16 17 18 19 20 21 13 14 15 16 17 18❤ 19 Sept 21 18 19 20 21 22 (23) 24 22 23 24 25 26X 27 28 20 21 22 23 24 25X 26 ❤ Oct 16 27SD 28SD 29SD 30SD 25 26❤ 31 29 30 27 28 29 30 31 Nov 16 Dec 14 January 2021 February 2021 March 2021 Jan 18 Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Sun Mon Tue Wed Thur Fri Sat Feb 20 1X 2 1 2 3 4 5 6 1 2 3 4 5 6 Mar 18 3 /4/ [5] 6❤ 7 8 9 7 8 9 10 11 12 13 7 8 9 10 11 (12)❤ 13 Apr 22 10 11 -

CALENDAR YEAR 2017 START DATE END DATE January 9, 2017
CALENDAR YEAR 2017 START DATE END DATE January 9, 2017 March 25, 2017 February 16, 2011 March 25, 2017 Mid-Term Start April 3, 2017 June 17, 2017 May 11, 2017 June 17, 2017 Mid-Term Start July 10, 2017 September 23, 2017 August 17, 2017 September 23, 2017 Mid-Term Start October 2, 2017 December 16, 2017 November 9, 2017 December 16, 2017 Mid-Term Start AiV START DATE END DATE January 2, 2017 March 19, 2017 February 19, 2017 March 19, 2017 Mid-Term Start April 3, 2017 June 18, 2017 May 11, 2017 June 18, 2017 Mid-Term Start July 3, 2017 September 17, 2017 August 10, 2017 September 17, 2017 Mid-Term Start October 2, 2017 December 17, 2017 November 9, 2019 December 17, 2017 Mid-Term Start CALENDAR YEAR 2018 START DATE END DATE January 8, 2018 March 24, 2018 February 15, 2018 March 24, 2018 Mid-Term Start April 2, 2018 June 16, 2018 May 10, 2018 June 16, 2018 Mid-Term Start July 9, 2018 September 22, 2018 August 16, 2018 September 22, 2018 Mid-Term Start October 1, 2018 December 15, 2018 November 8, 2018 December 15, 2018 Mid-Term Start AiV START DATE END DATE January 1, 2018 March 18, 2018 February 8, 2018 March 18, 2018 Mid-Term Start April 2, 2018 June 17, 2018 May 10, 2018 June 17, 2018 Mid-Term Start July 2, 2018 September 16, 2018 August 9, 2018 September 16, 2018 Mid-Term Start October 1, 2018 December 16, 2018 November 8, 2018 December 16, 2018 Mid-Term Start CALENDAR YEAR 2019 START DATE END DATE January 7, 2019 March 23, 2019 February 14, 2019 March 23, 2019 Mid-Term Start April 1, 2019 June 15, 2019 May 9, 2019 June 15, 2019 Mid-Term -

2016 7 Day Working Days Calendar
2016 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2016 306 Saturday, January 2, 2016 307 Sunday, January 3, 2016 308 Monday, January 4, 2016 309 Tuesday, January 5, 2016 310 Wednesday, January 6, 2016 311 Thursday, January 7, 2016 312 Friday, January 8, 2016 313 Saturday, January 9, 2016 314 Sunday, January 10, 2016 315 Monday, January 11, 2016 316 Tuesday, January 12, 2016 317 Wednesday, January 13, 2016 318 Thursday, January 14, 2016 319 Friday, January 15, 2016 320 Saturday, January 16, 2016 321 Sunday, January 17, 2016 322 Monday, January 18, 2016 323 Tuesday, January 19, 2016 324 Wednesday, January 20, 2016 325 Thursday, January 21, 2016 326 Friday, January 22, 2016 327 Saturday, January 23, 2016 328 Sunday, January 24, 2016 329 Monday, January 25, 2016 330 Tuesday, January 26, 2016 331 Wednesday, January 27, 2016 332 Thursday, January 28, 2016 333 Friday, January 29, 2016 334 Saturday, January 30, 2016 335 Sunday, January 31, 2016 336 Monday, February 1, 2016 337 Tuesday, February 2, 2016 338 Wednesday, February 3, 2016 339 Thursday, February 4, 2016 340 Date Number of the Calendar Date Friday, February 5, 2016 341 Saturday, February 6, 2016 342 Sunday, February