THE AGA KHAN UNIVERSITY Faculty of Health Sciences Department Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abbott Laboratories (Pak) Ltd. List of Non CNIC Shareholders Final Dividend for the Year Ended Dec 31, 2015 SNO WARRANT NO FOLIO NAME HOLDING ADDRESS 1 510004 95 MR
Abbott Laboratories (Pak) Ltd. List of non CNIC shareholders Final Dividend For the year ended Dec 31, 2015 SNO WARRANT_NO FOLIO NAME HOLDING ADDRESS 1 510004 95 MR. AKHTER HUSAIN 14 C-182, BLOCK-C NORTH NAZIMABAD KARACHI 2 510007 126 MR. AZIZUL HASAN KHAN 181 FLAT NO. A-31 ALLIANCE PARADISE APARTMENT PHASE-I, II-C/1 NAGAN CHORANGI, NORTH KARACHI KARACHI. 3 510008 131 MR. ABDUL RAZAK HASSAN 53 KISMAT TRADERS THATTAI COMPOUND KARACHI-74000. 4 510009 164 MR. MOHD. RAFIQ 1269 C/O TAJ TRADING CO. O.T. 8/81, KAGZI BAZAR KARACHI. 5 510010 169 MISS NUZHAT 1610 469/2 AZIZABAD FEDERAL 'B' AREA KARACHI 6 510011 223 HUSSAINA YOUSUF ALI 112 NAZRA MANZIL FLAT NO 2 1ST FLOOR, RODRICK STREET SOLDIER BAZAR NO. 2 KARACHI 7 510012 244 MR. ABDUL RASHID 2 NADIM MANZIL LY 8/44 5TH FLOOR, ROOM 37 HAJI ESMAIL ROAD GALI NO 3, NAYABAD KARACHI 8 510015 270 MR. MOHD. SOHAIL 192 FOURTH FLOOR HAJI WALI MOHD BUILDING MACCHI MIANI MARKET ROAD KHARADHAR KARACHI 9 510017 290 MOHD. YOUSUF BARI 1269 KUTCHI GALI NO 1 MARRIOT ROAD KARACHI 10 510019 298 MR. ZAFAR ALAM SIDDIQUI 192 A/192 BLOCK-L NORTH NAZIMABAD KARACHI 11 510020 300 MR. RAHIM 1269 32 JAFRI MANZIL KUTCHI GALI NO 3 JODIA BAZAR KARACHI 12 510021 301 MRS. SURRIYA ZAHEER 1610 A-113 BLOCK NO 2 GULSHAD-E-IQBAL KARACHI 13 510022 320 CH. ABDUL HAQUE 583 C/O MOHD HANIF ABDUL AZIZ HOUSE NO. 265-G, BLOCK-6 EXT. P.E.C.H.S. KARACHI. -
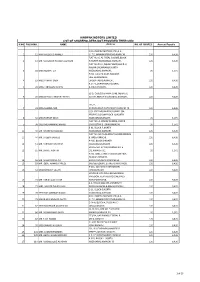
Hinopak Motors Limited List of Shareholders Not Provided Their Cnic S.No Folio No
HINOPAK MOTORS LIMITED LIST OF SHAREHOLDERS NOT PROVIDED THEIR CNIC S.NO FOLIO NO. NAME Address NO. OF SHARES Amount Payable C/O HINOPAK MOTORS LTD.,D-2, 1 12 MIR MAQSOOD AHMED S.I.T.E.,MANGHOPIR ROAD,KARACHI., 120 6,426 FLAT NO. 6, AL-FAZAL SQUARE,BLOCK- 2 13 MR. MANZOOR HUSSAIN QURESHI H,NORTH NAZIMABAD,KARACHI., 120 6,426 FLAT NO.19-O, IQBAL PLAZA,BLOCK-O, NAGAN CHOWRANGI,NORTH 3 18 MISS NUSRAT ZIA NAZIMABAD,KARACHI., 20 1,071 H.NO. E-13/40,NEAR RAILWAY LINE,GHARIBABAD, 4 19 MISS FARHAT SABA LIAQUATABAD,KARACHI., 120 6,426 R.177-1,SHARIFABADFEDERAL 5 24 MISS TABASSUM NISHAT B.AREA,KARACHI., 120 6,426 52-D, Q-BLOCK,PAHAR GANJ, NEAR LAL 6 28 MISS SHAKILA ANWAR FATIMA KOTTHI,NORTH NAZIMABAD,KARACHI., 120 6,426 171/2, 7 31 MISS SAMINA NAZ AURANGABAD,NAZIMABAD,KARACHI-18. 120 6,426 C/O. SYED MUJAHID HUSSAINP-394, PEOPLES COLONYBLOCK-N, NORTH 8 32 MISS FARHAT ABIDI NAZIMABADKARACHI, 20 1,071 FLAT NO. A-3FARAZ AVENUE, BLOCK- 9 38 SYED MOHAMMAD HAMID 20GULISTAN-E-JOHARKARACHI, 20 1,071 B-91, BLOCK-P,NORTH 10 40 MR. KHURSHID MAJEED NAZIMABAD,KARACHI. 120 6,426 FLAT NO. M-45,AL-AZAM SQUARE,FEDRAL 11 44 MR. SALEEM JAWEED B. AREA,KARACHI., 120 6,426 A-485, BLOCK-DNORTH 12 51 MR. FARRUKH GHAFFAR NAZIMABADKARACHI. 120 6,426 HOUSE NO. D/401,KORANGI NO. 5 13 55 MR. SHAKIL AKHTAR 1/2,KARACHI-31. 20 1,071 H.NO. 3281, STREET NO.10,NEW FIDA HUSSAIN SHAIKHA 14 56 MR. -

Agriauto Industries Limited List of Without Cnic Physical Shareholders As of 15/09/2015
AGRIAUTO INDUSTRIES LIMITED LIST OF WITHOUT CNIC PHYSICAL SHAREHOLDERS AS OF 15/09/2015 S.NO. FOLIO # NAME RELATION ADDRESS1 ADDRESS2 ADDRESS3 ADDRESS4 1 13 SHAHRUKH HASAN M. A. HASSAN 538/15, GARDEN EAST, KARACHI. 2 18 MOHAMMAD SHAFIQUE ULFAT HUSSAIN E-16, AL-NASEER SQUARE, FEDERAL B. AREA, KARACHI. 3 28 JUNAID AHMAD MR. SHAHID AHMAD C-175, K.D.A. SCHEME NO. 1-A, KARACHI. 4 33 MATLOOB ALI MASHOOQ ALI 28-B, BLOCK-15, OPP: MEHRAN EXTENSION APT., GULSHAN-E-IQBAL, KARACHI. 5 35 PARVEZ ALI MATLOOB ALI 28-B, BLOCK-15, OPP: MEHRAN EXTENSION APT., GULSHAN-E-IQBAL, KARACHI. 6 37 MOHAMMAD YASEEN ABUBAKER ABUBAKER USMAN 3/4, RIMPA TWIN STAR, OPP: KASHIF CENTRE, SHAHRAH-E-FAISAL, KARACHI. 7 38 JAMILA ABUBAKER ABUBAKER USMAN 3/4, RIMPA TWIN STAR, OPP: KASHIF CENTRE, SHAHRAH-E-FAISAL, KARACHI. 8 39 ABIDA ABUBAKER ABUBAKER USMAN 3/4, RIMPA TWIN STAR, OPP: KASHIF CENTRE, SHAHRAH-E-FAISAL, KARACHI. 9 40 FEROZA ABUBAKER ABUBAKER USMAN 3/4, RIMPA TWIN STAR, OPP: KASHIF CENTRE, SHAHRAH-E-FAISAL, KARACHI. 10 41 SAGHIR AHMAD FARUQI LATE DR. M. MASOOD FARUQI A-40, I/I, 3RD GIZRI STREET, PHASE IV, DEFENCE HOUSING AUTHORITY, KARACHI. 11 42 SAGHIR AHMAD KHAN NISAR AHMAD KHAN 56, BANGLORE TOWN, KARACHI-8. 12 44 WARIS ALI NANJI A/38, U.K. APPARTMENTS, UNIVERSITY ROAD, BLOCK-14, GULSHAN-E-IQBAL, KARACHI. 13 47 NASERA AFZAL AFZAL RASHID B/84/4, K.D.A. SCHEME-1, EXTENSION, OPP: MASJID-E-UZMA, KARACHI. 14 51 SUGHRA BEGUM GHULAM MOHAMMAD 53-D, GARDEN ROAD, KARACHI. -
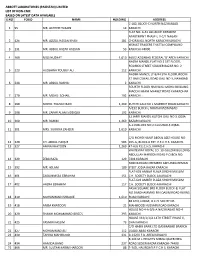
Abbott Laboratories (Pakistan) Limited List of Non-Cnic Based on Latest Data Available S.No Folio Name Holding Address 1 95
ABBOTT LABORATORIES (PAKISTAN) LIMITED LIST OF NON-CNIC BASED ON LATEST DATA AVAILABLE S.NO FOLIO NAME HOLDING ADDRESS C-182, BLOCK-C NORTH NAZIMABAD 1 95 MR. AKHTER HUSAIN 14 KARACHI FLAT NO. A-31 ALLIANCE PARADISE APARTMENT PHASE-I, II-C/1 NAGAN 2 126 MR. AZIZUL HASAN KHAN 181 CHORANGI, NORTH KARACHI KARACHI. KISMAT TRADERS THATTAI COMPOUND 3 131 MR. ABDUL RAZAK HASSAN 53 KARACHI-74000. 4 169 MISS NUZHAT 1,610 469/2 AZIZABAD FEDERAL 'B' AREA KARACHI NAZRA MANZIL FLAT NO 2 1ST FLOOR, RODRICK STREET SOLDIER BAZAR NO. 2 5 223 HUSSAINA YOUSUF ALI 112 KARACHI NADIM MANZIL LY 8/44 5TH FLOOR, ROOM 37 HAJI ESMAIL ROAD GALI NO 3, NAYABAD 6 244 MR. ABDUL RASHID 2 KARACHI FOURTH FLOOR HAJI WALI MOHD BUILDING MACCHI MIANI MARKET ROAD KHARADHAR 7 270 MR. MOHD. SOHAIL 192 KARACHI 8 290 MOHD. YOUSUF BARI 1,269 KUTCHI GALI NO 1 MARRIOT ROAD KARACHI A/192 BLOCK-L NORTH NAZIMABAD 9 298 MR. ZAFAR ALAM SIDDIQUI 192 KARACHI 32 JAFRI MANZIL KUTCHI GALI NO 3 JODIA 10 300 MR. RAHIM 1,269 BAZAR KARACHI A-113 BLOCK NO 2 GULSHAD-E-IQBAL 11 301 MRS. SURRIYA ZAHEER 1,610 KARACHI C/O MOHD HANIF ABDUL AZIZ HOUSE NO. 12 320 CH. ABDUL HAQUE 583 265-G, BLOCK-6 EXT. P.E.C.H.S. KARACHI. 13 327 AMNA KHATOON 1,269 47-A/6 P.E.C.H.S. KARACHI WHITEWAY ROYAL CO. 10-GULZAR BUILDING ABDULLAH HAROON ROAD P.O.BOX NO. 14 329 ZEBA RAZA 129 7494 KARACHI NO8 MARIAM CHEMBER AKHUNDA REMAN 15 392 MR. -

Agriauto Industries Limited List of Noncnic Interim Dividend 2016 For
AGRIAUTO INDUSTRIES LIMITED INTERIM DIVIDEND 50% RS. 2.50 PER SHARE FOR THE YEAR ENDING : JUNE 30, 2016 LIST OF NON‐CNIC CASES Name of Shareholder Address No. of Shares Held Net Dividend Payable SHAHRUKH HASAN 538/15, GARDEN EAST, KARACHI. 162 334.00 E‐16, AL‐NASEER SQUARE, FEDERAL B. MOHAMMAD SHAFIQUE AREA, KARACHI. 1,456 3,003.00 JUNAID AHMAD C‐175, K.D.A. SCHEME NO. 1‐A, KARACHI. 120 247.00 28‐B, BLOCK‐15, OPP: MEHRAN EXTENSION MATLOOB ALI APT., GULSHAN‐E‐IQBAL, KARACHI. 520 1,072.00 28‐B, BLOCK‐15, OPP: MEHRAN EXTENSION PARVEZ ALI APT., GULSHAN‐E‐IQBAL, KARACHI. 582 1,200.00 3/4, RIMPA TWIN STAR, OPP: KASHIF MOHAMMAD YASEEN ABUBAKER CENTRE, SHAHRAH‐E‐FAISAL, KARACHI. 33 68.00 3/4, RIMPA TWIN STAR, OPP: KASHIF JAMILA ABUBAKER CENTRE, SHAHRAH‐E‐FAISAL, KARACHI. 33 68.00 3/4, RIMPA TWIN STAR, OPP: KASHIF ABIDA ABUBAKER CENTRE, SHAHRAH‐E‐FAISAL, KARACHI. 8 16.00 3/4, RIMPA TWIN STAR, OPP: KASHIF FEROZA ABUBAKER CENTRE, SHAHRAH‐E‐FAISAL, KARACHI. 128 264.00 A‐40, I/I, 3RD GIZRI STREET, PHASE IV, SAGHIR AHMAD FARUQI DEFENCE HOUSING AUTHORITY, KARACHI. 20 41.00 SAGHIR AHMAD KHAN 56, BANGLORE TOWN, KARACHI‐8. 33 68.00 A/38, U.K. APPARTMENTS, UNIVERSITY ROAD, BLOCK‐14, GULSHAN‐E‐IQBAL, WARIS ALI KARACHI. 60 124.00 B/84/4, K.D.A. SCHEME‐1, EXTENSION, NASERA AFZAL OPP: MASJID‐E‐UZMA, KARACHI. 33 68.00 SUGHRA BEGUM 53‐D, GARDEN ROAD, KARACHI. 68 140.00 42/II, 6TH COMMERCIAL STREET, PHASE‐IV, PAKISTAN DEFENCE OFFICER'S HOUSING MIAN ASGHAR ALI AUTHORITY, KARACHI. -

Annual Report 2015
World Memon Organisation The Mission of the World Memon Organisation Charitable Foundation (“the WMO”) is to act as the central Memon Organisation representing the Memon community throughout the world and to promote the advancement, upliftment, unity, welfare and well-being of all Memons in particular and all Muslims in general in the world in all aspects of life and at all times in accordance with and under the guidance of Islamic principles. YOU CREATED A BRIGHTER FUTURE In 2015, You shaped the lives of thousands of children by providing them free education in Pakistan. through YOU MADE DREAMS COME TRUE A Hundred underprivileged families in India got a roof over their head in the Ahmedabad housing project, Gujarat. your YOU GAVE HOPE You delivered meals, shoes, warm clothing, medicines to a sea of generosity, humanity migrating from Syria into Europe. YOU MADE A DIFFERENCE You stood up against Xenophobia in South Africa, delivered aid all of this to flood victims in Malawi. Provided funds and water to children reeling under the heat and drought in Africa. was made YOU CHANGED LIVES You Empowered Women across the Globe. possible. YOU SHOWED YOU CARED You fed the fasting for the entire month of Ramadan in the Middle East. Thank You! Serving Mankind World Memon Organisation Annual Report 2015 ANNUAL REPORT 2015 | WORLD MEMON ORGANISATION 3 Content Office Bearers of WMO for the period 2014-2017 5 Message from the Chairman Board of Trustees – Mr. Haroon Karim 6 Message from President of WMO – Mr. Suliman Noor Mahomed (Solly Noor) 7 Notice & Agenda of 14th Annual General Assembly Meeting 8 Minutes of the 13th Annual General Assembly Meeting held on 01st September 2015 9 a. -

Abbott Laboratories (Pakistan) Limited List of Non-Cnic As at May 13, 2019 S.No Folio Name Address Holding 1 95 Mr
ABBOTT LABORATORIES (PAKISTAN) LIMITED LIST OF NON-CNIC AS AT MAY 13, 2019 S.NO FOLIO NAME ADDRESS HOLDING 1 95 MR. AKHTER HUSAIN C-182, BLOCK-C 14 NORTH NAZIMABAD KARACHI 2 126 MR. AZIZUL HASAN KHAN FLAT NO. A-31 181 ALLIANCE PARADISE APARTMENT PHASE-I, II-C/1 NAGAN CHORANGI, NORTH KARACHI KARACHI. 3 131 MR. ABDUL RAZAK HASSAN KISMAT TRADERS 53 THATTAI COMPOUND KARACHI-74000. 4 169 MISS NUZHAT 469/2 AZIZABAD 1,610 FEDERAL 'B' AREA KARACHI 5 223 HUSSAINA YOUSUF ALI NAZRA MANZIL FLAT NO 2 112 1ST FLOOR, RODRICK STREET SOLDIER BAZAR NO. 2 KARACHI 6 244 MR. ABDUL RASHID NADIM MANZIL LY 8/44 2 5TH FLOOR, ROOM 37 HAJI ESMAIL ROAD GALI NO 3, NAYABAD KARACHI 7 270 MR. MOHD. SOHAIL FOURTH FLOOR 192 HAJI WALI MOHD BUILDING MACCHI MIANI MARKET ROAD KHARADHAR KARACHI 8 290 MOHD. YOUSUF BARI KUTCHI GALI NO 1 1,269 MARRIOT ROAD KARACHI 9 298 MR. ZAFAR ALAM SIDDIQUI A/192 BLOCK-L 192 NORTH NAZIMABAD KARACHI 10 300 MR. RAHIM 32 JAFRI MANZIL 1,269 KUTCHI GALI NO 3 JODIA BAZAR KARACHI 11 301 MRS. SURRIYA ZAHEER A-113 BLOCK NO 2 1,610 GULSHAD-E-IQBAL KARACHI 12 320 CH. ABDUL HAQUE C/O MOHD HANIF ABDUL AZIZ 583 HOUSE NO. 265-G, BLOCK-6 EXT. P.E.C.H.S. KARACHI. 13 327 AMNA KHATOON 47-A/6 1,269 P.E.C.H.S. KARACHI 14 329 ZEBA RAZA WHITEWAY ROYAL CO. 129 10-GULZAR BUILDING ABDULLAH HAROON ROAD P.O.BOX NO. -

JUBILEE LIFE INSURANCE COMPANY LIMITED List of Physical Shareholders As of 03-03-0214 S
JUBILEE LIFE INSURANCE COMPANY LIMITED List of Physical Shareholders as of 03-03-0214 S. No. Folio No. Security Holder Name Address 1 11 MR. MOHAMMAD ABDULLAH B-31, K.D.A./1, KARACHI. 2 14 DR. FAZAL M. ABREJO C-41, I.T. OFFICER S COLONY, GARDEN, KARACHI. 3 23 MR. EJAZ AHED D-222/2, K.D.A. SCHEME NO.1-A EXTENSION, KARACHI-75350. 4 25 MR. M. MAHBOOB AHMAD 132, UPPER MALL SCHEME, LAHORE. 5 27 MR. RAFI-UDDEEN AHMAD H.3-A, ST-32, F-8/1, ISLAMABAD. 6 28 SYED NASEEM AHMAD HOUSE NO.3-A, FIRST WEST STREET, DEFENCE HOUSING AUTHORITY, PHASE-1, KARACHI. 7 29 MR. BEHRAM K. AHMED 49/1/II, KHYABAN-I-GHAZI, PHASE-5, D.H.S., KARACHI. 8 35 MR. ISAR AHMAD 11, BATHVIEW APARTMENTS, G-25, BLOCK-9, KHAYABAN-E-JAMI CLIFTON, KARACHI. 9 36 MR. ISMAIL H. AHMED 71/II, 10TH STREET, PHASE-VI, KHAYABAN-E-BADAR, D.H.A., KARACHI 10 39 MR. MASOOD AHMED 164/A, F-10/1, STREET # 36, ISLAMABAD. 11 41 MR. MOHD SIDDIQ AHMED C/O AMERICAN PRESIDENT LINES, EBRAHIM BUILDING, WEST WHARF, KARACHI. 12 42 MRS. AISHA AHMED SUITE 406-408,4TH FLOOR, AL-FALAH BUILDING, SHAHRAH-E-QUAID-E-AZAM, LAHORE. 13 43 MR. MUMTAZ AHMED 74/1-A, LALAZAR, M.T. KHAN ROAD, KARACHI. 14 48 MR. SULTAN AHMED D-42, BLOCK-IV, KEHKASHAN CLIFTON, KARACHI-75600. 15 71 SYED AKBAR ALIKHAN F-2, BLOCK-4, CLIFTON, KARACHI. 16 72 MR. ZAFAR ALTAF HOUSE NO.182, ST.97, SECTOR I-8/4, ISLAMABAD. -
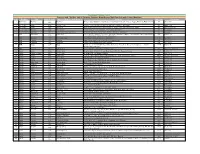
Province and City Wise List of Saturday Operative Branches And
BankIslami Pakistan Limited Province and City Wise List of Saturday Operative Branches and Sub Branches with Contact Numbers S.No Province City Branch Code Name of Branch / Sub Branch Address City Code Phone No 1 Balochistan Khanozai Khanozai Khasra # 1533, Khatooni # 143, Khewat # 135, Mutation No.472, Mouza-e-Tappa, Khanozai, District Pishin. 082 1053 6427250-51 2 Balochistan Quetta 1070 Satellite Town Shop No.3, Dead Karez, Opposite Old Bus Adda, Satellite Town, Quetta 081 2448701-04 3 Balochistan Quetta 1160 Suraj Ganj Khasra # 148,144/1,148/1,1753/17, Ward # 35, Tappa Urban, Suraj Ganj Bazar, Quetta 081 840089-90-91-94 4 Federal Capital Islamabad 3050 F-11 Markaz Plot No.14, Near PTCL Exchange, F-11 Markaz, Islamabad 051 2111456-58 5 Federal Capital Islamabad 3058 Razia Sharif Plaza, Blue Area, 90 West, Razia Sharif Plaza, Jinnah Avenue, Blue Area, Islamabad 051 2802248 6 KPK Abbottabad Abbottabad Plot # 195-207, Khasra # 2302-2305, Abbottabad Business Complex, Moza Shaikul Bandi, Amir Shaheed 099 3010 2343959-64 Road, Supply Bazar, Manshera Road, Abbottabad. 7 KPK D.I. Khan 3019 Dera Ismail Khan Tank Adda, Circular Road, Dera Ismail Khan. 0966 73026563 8 KPK Mingora 3025 Mingora Main Madyan Road, Near Greek Chowk, Mingora, Swat. 094 6710386-91 9 KPK Timargara 3027 Timargara Gurguri Chowk, Balambad Road, Timargara. 094 5825607-10 10 KPK Naran 3037 Naran Plot Khasra No.71/1185,MNJ Road, Main Bazar,Near China Store, Naran, Moza Kaghan, Tehsil '0997 430261-64 Balakot, District Mansehra. 11 KPK Peshwar 3066 Cantt Branch AYS Centre No, 3 Arbab Road, Peshwar Cantt 091 5830026 & 5830028 12 Punjab Lahore 2004 Circular Road Shop No.S-38, R-105/H, Circular Road, Outside Mochee Darwaza, Lahore 0423 7639041-42 13 Punjab Rahim Yar Khan 2009 Model Town 21-A, Model Town, Rahim Yar Khan 068 5886971-78 14 Punjab Lahore 2016 Azam Cloth F1207/A, Chuna Mandi, Adjacent Mohallah Sareen, Azam Cloth Market, Lahore 0423 7670188 & 7670256 15 Punjab Sahiwal 2017 Jinnah Road 418, High Street, Jinnah Road, Sahiwal. -

To Open Bankislami Pakistan Limited Details
S.No Branch Name Address City Province Supply Bazar 1 Plot # 195-207, Khasra # 2302-2305, Abbottabad Business Complex, Moza Shaikul Bandi, Amir Shaheed Road, Supply Bazar, Manshera Road, Abbottabad. Abbottabad KPK 2 Arifwala Plot No.115, H-Block, Thana Bazar, Arifwala Arifwala Punjab 3 Attock Omair Arcade, Opposite Peoples Colony, Main Attock Road, Attock. Attock Punjab 4 Ghourghusti Miskeenabad, Ghourghusti, Tehsil Hazro, District Attock Attock Punjab 5 Badin Mani Quaid-e-azam Road, Near Qazia Wah, Badin Badin Sindh 6 Golarchi Plot No. A-4, Golarchi Town, Taluka Shaheed Fazil Rao, District Badin Badin Sindh 7 Satellite Town Plot # 53-C, Commercial Area, Satellite Town, Bahawalpur Bahawalpur Punjab 8 Circular Road Block No. 915, Circular Road Bahawalpur. Bahawalpur Punjab 9 Balakot Plot, Khasra No.3626/1046, Moza Balakot,Tehsil Balakot, District Mansehra Balakot KPK 10 Batagram Khasra No.792, Moza Ajmairah, Tehsil & District Batgram. Batagram KPK 11 Batkhela Main Bazar Batkhela, Tehsil Sawat Ranizai, District Malakand Batkhela KPK 12 Beesham Plot Khasra No.583, Moza Butyal, Main Road Besham, Tehsil Besham, District Shangla Beesham KPK 13 Booni Booni Bazar, Village & P.O Booni, Tehsil Mastaj, District Chitral Booni KPK 14 Chaksawari, AJK Main Mirpur Road, Near Attock Petrol Pump, Chaksawari, District Mirpur, Azad Kashmir Chaksawari AJK 15 Chakwal Khasra # 4516 Jhelum Road Chakwal. Chakwal Punjab 16 Chaman Khasra # 208 & 209, Mall Road, Chaman Chaman Balochistan 17 Chichawatni Plot No.146, Khatooni # 239, G.T. Road, Chichawatni Chichawatni Punjab 18 Chilas Main Bazar, DC Chowk, Rani Road Chillas, District Diamer. Chilas KPK 19 Chiniot 1- A Shahra-e- Quaid Azam , Chiniot. -

Integrating Recycling and Disposal System for Solid Waste Management in Karachi
INTEGRATING RECYCLING AND DISPOSAL SYSTEM FOR SOLID WASTE MANAGEMENT IN KARACHI By Dr. Mansoor Ali Arif Hasan (Final Draft, June 20, 2001) Field work and technical support: Engr. Mansoor Raza and the Urban Resource Centre, Karachi Arif Hasan, Architect and Planning Consultant, 37-D, Mohd. Ali Society, Karachi – 75350 – Pakistan Tel/Fax: (92.21) 452 2361 E-mail: [email protected] ACKNOWLEDGEMENTS This report is the culmination of many years of work on solid waste management by Dr. Mansoor Ali and the Urban Resource Centre (URC). Its background and methodology is explained in the introduction. However, how this work began needs to be explained. In 1992, Dr. Mansoor Ali was doing his Master’s thesis at the University of Loughborough on solid waste and its relation to the recycling industry in Karachi. He sought the help of the URC for field investigations. The URC remained in touch with Dr. Mansoor Ali and continued this work around one question: Why does solid waste not reach the landfill site? And the answer invariably was that it was because of the requirements and location of the recycling industry and the scavenging system that served it. This led the URC to lobby for the creation of a “garbage city” where scavenging and recycling could be located. The Sindh Governor’s Task Force for Municipal Services accepted this concept for investigation and discussion purposes and after negotiations with interest groups, endorsed it. The logical conclusion of this endorsement was to prepare a pre-feasibility report and hence this study. Meanwhile, Dr. Mansoor over the years had continued to work on solid waste management issues related to Karachi. -

Aba Umar Dada Abdul Aziz Kaya
Memon Personalities Aba Umar Dada Late Mr. Dada was a well-known community leader and social worker. He was a prominent member of Karachi Cotton Exchange who earned a name for himself. After the establishment of Pakistan, he settled in the interior of Sindh and took leading part in all social and welfare activities of Hyderabad and Sindh. Settling in Karachi, he continued with his social work and was very active amongst the leaders of the Pakistan Memon Federation. Ahmed H.A. Dada He was a very prominent businessman and an active member of Karachi Stock Exchange rising to the post of its President. He was also on the Local Advisory Committee of National Bank of Pakistan, Karachi Branch, and was popular in the business circles. Abdul Aziz Kaya While in Hyderabad Deccan, he joined Ittehadul Muslimeen under the leadership of Mr. Qasim Rizvi. He worked very actively for the victims of the Indian an-ny. In Karachi, on the advice of Pakistan Ambassador Haji A. Sattar Seth, he was asked to infomi all the Hujjaj about the aims and objects of the creation of Pakistan and as such Haji Aziz started his mission. Durino Haj he rendered noteworthy services to the Hujjaj. He remained involved with his business for a couple of decades and again started his social service activities and established many institutions through which he served the people. During political turmoil when Karachi was under constant curfew for several days at a stretch, he stored consumer products and food products which he supplied at concessive rates without any profit.