SHIGELLA Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cy Martin Collection
University of Oklahoma Libraries Western History Collections Cy Martin Collection Martin, Cy (1919–1980). Papers, 1966–1975. 2.33 feet. Author. Manuscripts (1968) of “Your Horoscope,” children’s stories, and books (1973–1975), all written by Martin; magazines (1966–1975), some containing stories by Martin; and biographical information on Cy Martin, who wrote under the pen name of William Stillman Keezer. _________________ Box 1 Real West: May 1966, January 1967, January 1968, April 1968, May 1968, June 1968, May 1969, June 1969, November 1969, May 1972, September 1972, December 1972, February 1973, March 1973, April 1973, June 1973. Real West (annual): 1970, 1972. Frontier West: February 1970, April 1970, June1970. True Frontier: December 1971. Outlaws of the Old West: October 1972. Mental Health and Human Behavior (3rd ed.) by William S. Keezer. The History of Astrology by Zolar. Box 2 Folder: 1. Workbook and experiments in physiological psychology. 2. Workbook for physiological psychology. 3. Cagliostro history. 4. Biographical notes on W.S. Keezer (pen name Cy Martin). 5. Miscellaneous stories (one by Venerable Ancestor Zerkee, others by Grandpa Doc). Real West: December 1969, February 1970, March 1970, May 1970, September 1970, October 1970, November 1970, December 1970, January 1971, May 1971, August 1971, December 1971, January 1972, February 1972. True Frontier: May 1969, September 1970, July 1971. Frontier Times: January 1969. Great West: December 1972. Real Frontier: April 1971. Box 3 Ford Times: February 1968. Popular Medicine: February 1968, December 1968, January 1971. Western Digest: November 1969 (2 copies). Golden West: March 1965, January 1965, May 1965 July 1965, September 1965, January 1966, March 1966, May 1966, September 1970, September 1970 (partial), July 1972, August 1972, November 1972, December 1972, December 1973. -

Review No. 11 - Alexander MILES V
Review No. 11 - Alexander MILES v. US - 2 August, 1971. ___________________________________________________<Prev___Next>__ IN THE MATTER OF MERCHANT MARINER'S DOCUMENT NO.Z-705010-D1 AND ALL OTHER SEAMAN'S DOCUMENTS Issued to:Alexander MILES Z-705010-D1 DECISION OF THE COMMANDANT UNITED STATES COAST GUARD 11 Alexander MILES This review has been taken in accordance with Title 46 United States Code 239(g) and Title 46 Code of Federal Regulations 137.35. By order dated 20 October 1969, an Examiner of the United States Coast Guard at New Orleans, La., suspended Appellant's seaman's documents for six months upon finding him guilty of misconduct. The specifications found proved allege that while serving as an able seaman on board SS DOCTOR LYKES under authority of the document above captioned, Appellant: (1) on 18 May 1969, at sea, failed to perform his duties; (2) on 2 and 3 July 1969, at Manila, P.R., failed to perform his assigned duties; and (3) on 6 July 1969, at Hong Kong, failed to perform duties by reason of intoxication. At the hearing, Appellant did not appear. The Examiner entered a plea of not guilty to the charge and each specification. The Investigating Officer introduced in evidence voyage records of DOCTOR LYKES. file:///S|/Suspension_Revocation/Reviews/D11908.htm (1 of 5)10/30/2008 12:41:54 PM Review No. 11 - Alexander MILES v. US - 2 August, 1971. There was no defense. At the end of the hearing, the Examiner rendered a written decision in which he concluded that the charge and specifications had been proved. -

COMPLAINT by ZAMBIA 411 at Its 1590Th Meeting, on 8 October 1971
COMPLAINT BY ZAMBIA 411 Decisions and also the letter from forty-seven Member States contained in document S/10364,46 At its 1590th meeting, on 8 October 1971, the Taking note of the statement of the Permanent Rep Council decided to invite the representatives of Zam resentative of Zambia concerning violations of the bia the United Republic of Tanzania, Nigeria, South sovereignty, air space and territorial integrity of Zam Af;ica, Kenya and Guinea to participate, without vote, bia by South Africa, 47 in the discussion of the item entitled: "Complaint by Taking note of the statement of the Minister for Zambia: letter dated 6 October 1971 from the Per Foreign Affairs of the Republic of South Africa,4 7 manent Representative of Zambia to the United Nations addressed to the President of the Security Council Bearing in mind that all Member States must refrain (S/10352)".46 in their relations from the threat or use of force against the territorial integrity or political independence of any State, Conscious that it has the responsibility to take effi cient collective measures to prevent and eliminate At its 1591st meeting, on 11 October 1971, the threats to peace and security, Council decided to invite the representatives of Yugo Concerned by the situation on the borders of Zam slavia, India and Pakistan to participate, without vote, bia and Namibia, in the vicinity of the Caprivi Strip, in the discussion of the question. l. Reiterates that any violation of the sovereignty and territorial integrity of a Member State is contrary to the Charter of the United Nations; 2. -

Notes on the Birds of Southampton Island, Northwest Territories
Notes on the Birds of Southampton Island, Northwest Territories GERALD R. PARKER'and R. KENYONROSS2 ABSTRACT. During thesummers of 1970 and 1971,46 species were seenon Southamp- ton Island, most in the interior of the island where previous records were scarce. A comparison with observations in 1932 suggestslittle change in thestatus of the avifauna of the island over the past 40 years. RÉSUMÉ: Notes sur les oiseaux deI'île de Southampton, Territoires du Nord-Ouest. Au cours des étés de 1970 et 1911, les auteurs ont aperçu sur l'île de Southampton 46 esphces, la plupart dans l'intérieur, où les mentions antérieures sont rares. La comparaison avec des observations de 1932 montre peu de changement dans l'état de l'avifaune de l'île au cours des'40 dernièresannées. PE3HI". ET eonpocy O nmuym ocmposa CagrnZemwnoH Cesepo-9anadnw Tep- PUmOpUU). B TeYeHHe JIeTHHX nepHonoB 1970 H 197lrr Ha OCTpOBe CayTreMIITOH 6~noSaMerfeHO 46 BIlnOB IlTIl4, FJIaBHbIM 06pa30~,BO BHYTPeHHefi YaCTH OCTPOBB, rge paHee perzwrpaqm EIX npoBoAHnacb peAIco. CpaBHeme c H~~JII~A~HEI~EI 1932rnoIcasmBaeT, YTO nTmbrx 4ayHa ocTposa Mano H~M~HEI~~CLsa nocnenme 40 neT. INTRODUCTION A barren-ground caribourange evaluation of Southampton Island, conducted by the Canadian Wildlife Service, provided the opportunity to observe the birds on the island during the periods 2 June to 14 August 1970 and 1 July to 31 August 1971. The main camp in 1970 was on the Southampton Limestone Plains of the Hudson Bay Lowlands (Bird 1953) at Salmon Pond (64" 14' N., 85" 00' W.), although several trips were made in July, 15 miles northeast to the Precambrian highlands. -
![Library Bulletin [International Planned Parenthood Federation, November 1972]](https://docslib.b-cdn.net/cover/2993/library-bulletin-international-planned-parenthood-federation-november-1972-362993.webp)
Library Bulletin [International Planned Parenthood Federation, November 1972]
ED 071 gad ts015 452 TITLE Bulletin (International'Manned Parenthood Federation, November 1972]. INSTITUTION International Planned Parenthood Federation, London (England). PUB DATE Nov 72 NOTE 70p. EDRS PRTC2 MF-$0.65 HC-S3.29 DESCRIPTORS Bibliographies; *Books; Classification;Demography; *Family planning; Indexes (I,ocateil);*Library _Collections; *Population Trends; Publications; . *Reference Materials . ABSTRACT Resources located in the International Planned Parenthood Federation (/PPF) HeadquartersLibrary are inventoried in this_quarterly library bulletin. Entries_follow.theIPPF classification scheme. and are arranged by topics:(1) .general office. managenent_and administration; (2)_ contraceptive methods;(3) family. _planning (general); (4) United Nations, conferencess_etc.; (5) Population -- Africa, America, Asia,.Europe, Oceania, U.S.S.R.; (6) religious, moral,. ethical, and politicaa.attitudes.to family planning; (7) medical; (8) education, includingsex and population education ;. and _(9) .communication,publicity, history, etc. The name of the publisher/supplier andBritish_currency price of the book are _supplied for.eacknOtation withan. alphabetical list of .publisher/isuppliersand their addresses appended. An alphabetical list of authors.is also included together . with classification numbers . .for cross-references. (BL) 1 International Planned Parenthood U S DEPARTMENT OF HEALTH. Federation EDUCATION & WELFARE 18 20 Lower Regent Street, OFFICE OF EDUCATION London SW1Y 4PW THIS DOCUMENT HAS BEENREPRO DUCED EXACTLY AS RECEIVEDFROM -

International Review of the Red Cross, November 1971, Eleventh Year
NOVEMBER 1971 ELEVENTH YEAR - No.128 international review• of the red cross PROPERTY OF U.S. ARMY l'HE JUDGE ADVOCATE GENERAl'S SCHOOl LtSAARY INTER ARMA CARITAS GENEVA INTERNATIONAL COMMITTEE OF THE RED CROSS FOUNDED IN 1863 INTERNATIONAL COMMITTEE OF THE RED CROSS MARCEL A. NAVILLE, President (member since 1967) JEAN PICTET, Doctor of Laws, Chairman of the Legal Commission, Vice-President (1967) HARALD HUBER, Doctor of Laws, Federal Court judge, Vice-President (1969) PAUL RUEGGER, Ambassador, President of the ICRC from 1948 to 1955 (1948) GUILLAUME BORDIER, Certificated Engineer E.P.F., M.B.A. Harvard, Banker (1955) HANS BACHMANN, Doctor of Laws, Winterthur Stadtrat (1958) JACQUES FREYMOND, Doctor of Literature, Director of the Graduate Institute of International Studies, Professor at the University of Geneva (1959) DIETRICH SCHINDLER, Doctor of Laws, Professor at the University of Zurich (1961) MARJORIE DUVILLARD, Nurse (1961) MAX PETITPIERRE, Doctor of Laws, former President of the Swiss Confederation (1961) ADOLPHE GRAEDEL, member of the Swiss National Council from 1951 to 1963, former Secretary-General of the International Metal Workers Federation (1965) DENISE BINDSCHEDLER-ROBERT, Doctor of Laws, Professor at the Graduate Institute of International Studies (1967) JACQUES F. DE ROUGEMONT, Doctor of Medicine (1967) ROGER GALLOPIN, Doctor of Laws, former Director-General (1967) WALDEMAR JUCKER, Doctor of Laws, Secretary, Union syndicale suisse (1967) VICTOR H. UMBRICHT, Doctor of Laws, Managing Director (1970) PIERRE MICHELI (1971) Honorar" members: Mr. JACQUES CHENEVIERE, Honorar" Vice-President; Miss LUCIE ODIER, Honorar" Vice-President; Messrs. CARL j. BURCKHARDT, PAUL CARRY, Mrs. MARGUERITE GAUTIER-VAN BERCHEM, Messrs. SAMUEL A. -

COMPLAINT by ZAMBIA45 Decisions at Its 1590Th Meeting, on 8 October
COMPLAINT BY ZAMBIA45 Decisions and also the letter from forty-seven Member States contained in document S/10364,46 At its 1590th meeting, on 8 October 1971, the Taking note of the statement of the Permanent Rep Council decided to invite the representatives of Zam resentative of Zambia concerning violations of the bia, the United Republic of Tanzania, Nigeria, South sovereignty, air space and territorial integrity of Zam Africa, Kenya and Guinea to participate, without vote, bia by South Africa,47 in the discussion of the item entitled: "Complaint by Taking note of the statement of the Minister for Zambia: letter dated 6 October 1971 from the Per Foreign Affairs of the Republic of South Africa,47 manent Representative of Zambia to the United Nations addressed to the President of the Security Council Bearing in mind that all Member States must refrain (S/10352)".46 in their relations from the threat or use of force against the territorial integrity or political independence of any State, Conscious that it has the responsibility to take effi cient collective measures to prevent and eliminate At its 1591st meeting, on 11 October 1971, the threats to peace and security, Council decided to invite the representatives of Yugo Concerned by the situation on the borders of Zam slavia, India and Pakistan to participate, without vote, bia and Namibia, in the vicinity of the Caprivi Strip, in the discussion of the question. 1. Reiterates that any violation of the sovereignty and territorial integrity of a Member State is contrary to the Charter of the United Nations; 2. -

Official Report of Proceedings
HONG KONG LEGISLATIVE COUNCIL—17th November 1971. 195 OFFICIAL REPORT OF PROCEEDINGS Wednesday, 17th November 1971 The Council met at half-past Two o'clock [MR PRESIDENT in the Chair] PRESENT HIS EXCELLENCY THE ACTING GOVERNOR (PRESIDENT) SIR HUGH SELBY NORMAN-WALKER, KCMG, OBE, JP THE HONOURABLE THE COLONIAL SECRETARY (Acting) MR MICHAEL DENY'S ARTHUR CLINTON, CMG, GM*, JP THE HONOURABLE THE ATTORNEY GENERAL MR DENYS TUDOR EMIL ROBERTS, CBE, QC, JP THE HONOURABLE THE SECRETARY FOR HOME AFFAIRS MR DONALD COLLIN CUMYN LUDDINGTON, JP THE HONOURABLE THE FINANCIAL SECRETARY MR CHARLES PHILIP HADDON-CAVE, JP THE HONOURABLE DAVID RICHARD WATSON ALEXANDER, MBE, JP DIRECTOR OF URBAN SERVICES THE HONOURABLE JAMES JEAVONS ROBSON, JP DIRECTOR OF PUBLIC WORKS THE HONOURABLE JOHN CANNING, JP DIRECTOR OF EDUCATION DR THE HONOURABLE GERALD HUGH CHOA, JP DIRECTOR OF MEDICAL AND HEALTH SERVICES THE HONOURABLE JACK CATER, MBE, JP DIRECTOR OF COMMERCE AND INDUSTRY THE HONOURABLE DENTS CAMPBELL BRAY, JP DISTRICT COMMISSIONER, NEW TERRITORIES THE HONOURABLE PAUL TSUI KA-CHEUNG, OBE, JP COMMISSIONER OF LABOUR THE HONOURABLE TAN MACDONALD LIGHTBODY, JP COMMISSIONER FOR RESETTLEMENT THE HONOURABLE WOO PAK-CHUEN, OBE, JP THE HONOURABLE SZETO WAI, OBE, JP THE HONOURABLE WILFRED WONG SIEN-BING, OBE, JP THE HONOURABLE ELLEN LI SHU-PUI, OBE, JP THE HONOURABLE WILSON WANG TZE-SAM, OBE, JP THE HONOURABLE HERBERT JOHN CHARLES BROWNE, JP DR THE HONOURABLE CHUNG SZE-YUEN, OBE, JP THE HONOURABLE LEE QUO-WEI, OBE, JP THE HONOURABLE OSWALD VICTOR CHEUNG, QC, JP THE HONOURABLE GERALD MORDAUNT BROOME SALMON, JP THE HONOURABLE ANN TSE-KAI, OBE, JP THE HONOURABLE LO KWEE-SEONG, OBE, JP ABSENT THE HONOURABLE KAN YUET-KEUNG, CBE, JP IN ATTENDANCE THE CLERK TO THE LEGISLATIVE COUNCIL MR RODERICK JOHN FRAMPTON HONG KONG LEGISLATIVE COUNCIL—17th November 1971. -

3Rupture in South Asia
3Rupture in South Asia While the 1950s had seen UNHCR preoccupied with events in Europe and the 1960s with events in Africa following decolonization, the 1970s saw a further expansion of UNHCR’s activities as refugee problems arose in the newly independent states. Although UNHCR had briefly been engaged in assisting Chinese refugees in Hong Kong in the 1950s, it was not until the 1970s that UNHCR became involved in a large-scale relief operation in Asia. In the quarter of a century after the end of the Second World War, virtually all the previously colonized countries of Asia obtained independence. In some states this occurred peacefully,but for others—including Indonesia and to a lesser extent Malaysia and the Philippines—the struggle for independence involved violence. The most dramatic upheaval, however, was on the Indian sub-continent where communal violence resulted in partition and the creation of two separate states—India and Pakistan—in 1947. An estimated 14 million people were displaced at the time, as Muslims in India fled to Pakistan and Hindus in Pakistan fled to India. Similar movements took place on a smaller scale in succeeding years. Inevitably, such a momentous process produced strains and stresses in the newly decolonized states. Many newly independent countries found it difficult to maintain democratic political systems, given the economic problems which they faced, political challenges from the left and the right, and the overarching pressures of the Cold War. In several countries in Asia, the army seized political power in a wave of coups which began a decade or so after independence. -
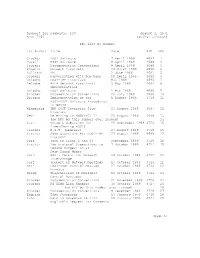
Request for Comments: 200 August 1, 1971 NIC: 7152 (Author Unknown) RFC List by Number 1St Author Title Date NI
Request for Comments: 200 August 1, 1971 NIC: 7152 (Author unknown) RFC List by Number 1st Author Title Date NIC RFC Crocker HOST Software 7 April 1969 4687 1 Duvall HOST Software 9 April 1969 4688 2 Crocker Documentation Conventions 9 April 1969 4689 3 Shapiro Network Timetable 24 March 1969 4690 4 Rulifson DEL 2 June 1969 4691 5 Crocker Conversation with Bob Kahn 10 April 1969 4692 6 Deloche HOST-IMP Interface May 1969 4693 7 Deloche ARPA Network Functional 5 May 1969 4694 8 Specifications Deloche HOST Software 1 May 1969 4695 9 Crocker Documentation Conventions 29 July 1969 4696 10 Deloche Implementation of the 1 August 1969 4718 11 HOST-HOST Software Procedures in GORDO Wingfield IMP-HOST Interface Flow 26 August 1969 4697 12 Diagrams Cerf Referring to NWG/RFC: 11 20 August 1969 4698 13 (no RFC by this number ever issued) 14 Carr Network Subsystem for 25 September 1969 4754 15 Time-Sharing HOSTS Crocker M.I.T. (address) 27 August 1969 4719 16 Kreznar Some Questions Re: HOST-IMP 27 August 1969 4699 17 Protocol Cerf (use of links 1 and 2) September 1969 4720 18 Kreznar Two Protocol Suggestions to 7 October 1969 4721 19 Reduce Congestion at Swap-Bound Nodes Cerf ASCII Format for Network 16 October 1969 4722 20 Interchange Cerf (report of Network meeting) 17 October 1969 4723 21 Cerf HOST-HOST Control Message 17 October 1969 4724 22 Formats Gregg Transmission of Multiple 16 October 1969 4725 23 Control Messages Crocker Documentation Conventions 21 November 1969 4726 24 Crocker No High Link Numbers 30 October 1969 4727 25 (no RFC by this number ever issued) 26 Crocker Documentation Conventions 9 December 1969 4729 27 English Time Standards 13 January 1970 4730 28 Kahn Note in Response to Bill 19 January 1970 4731 29 English's Request for Comments [Page 1] RFC 200 RFC List by Number August 1971 Crocker Documentation Conventions 4 February 1970 4732 30 Bobrow Binary Message Formats in February 1968 4733 31 Computer Network Vedder Connecting M.I.T. -

The Kentucky High School Athlete, August 1971 Kentucky High School Athletic Association
Eastern Kentucky University Encompass The Athlete Kentucky High School Athletic Association 8-1-1971 The Kentucky High School Athlete, August 1971 Kentucky High School Athletic Association Follow this and additional works at: http://encompass.eku.edu/athlete Recommended Citation Kentucky High School Athletic Association, "The Kentucky High School Athlete, August 1971" (1971). The Athlete. Book 163. http://encompass.eku.edu/athlete/163 This Article is brought to you for free and open access by the Kentucky High School Athletic Association at Encompass. It has been accepted for inclusion in The Athlete by an authorized administrator of Encompass. For more information, please contact [email protected]. HighSchoolAthMe DAVIESS COUNTY HIGH SCHOOL BASEBALL TEAM K.H.S.A.A. CHAMPION— 1971 (Left to Right) Front Row: C. Truman, G. Jones, D. Crowe, D, Zuerner, J. Rhinerson, J. Baker, G. Taylor. Second Row: Coach R. Embry, R. Hen- dricks, N. Lively, E. McCoUam, J. Ralph, J. Church, J. Ferrell, R. Acton, J. Hodskins. Official Organ of the KENTUCKY HIGH SCHOOL ATHLETIC ASSOCIATION AUGUST 1971 — ——— — — — ——— KENTUCKY HIGH SCHOOL TRACK MEET—CLASS AAA Louisville, Kenlucky, May 21, 1971 Liniis\illc Male HiL;li Sclnxil Track Team—K.H.S.A.A. Champion 19^1 ILell tc Right! Fronl How: R. Buller, L. Tennyson, R. Carpprter, W'. Gordon, C. Childers, J. While, C. Smilh Second Row: K. Waller, H. Crawford, M. Long, G. Sanderson, E. Gray, B. Sistrunk, K, Walkins. Dorsey, G. Wise Third Row: D. Calfcy, D. Thornton, J. Caffey, D. Hickersor. O, Gaines, T. Thomas, R, Robertson. Fourth Row: E. Hill, E. Murphy, M. -

M#-' FEDERAL RESERVE
FEDERAL RESERVE - m#-' statistical release For immediate release January 12, 1973 AUTOMOBILE LOANS BY MAJOR FINANCE COMPANIES LENDING TERMS TIGHTENED SOMEWHAT IN THE USED CAR MARKET AS THE PROPORTION OF USED CAR CONTRACTS WITH MATURITIES OVER 24 MONTHS DECLINED AGAIN DURING NOVEMBER. 1970 1971 1972 MATURITY DISTRIBUTION - NEW CAR CONTRACTS 24 months 25-30 31-36 Over 36 Balloon months months months Total 7c % 7. % 7. Seasonally adjusted November 1972 12.4 3.0 83.5 1.3 0.3 100.0 October 1972 12.9 2.7 83.4 1.2 0.2 100.0 September 1972 11.9 2.3 84.0 1.3 . 0.3 100.0 August 1972 11.9 2.2 84.2 0.9 0.3 100.0 Unadjusted November 1972 14.3 3.3 80.8 1.3 0.4 100.0 October 1972 14.5 2.8 81.4 1.1 0.2 100.0 November 1971 15.3 3 .0 81.2 0.2 0.3 100.0 MATURITY DISTRIBUTION - USED CAR CONTRACTS 18 months 19-24 25-30 Over 30 Balloon months months months Total P 70 70 % 7. % Seasonally adjusted November 1972 5.1 15.8 i 38.5 41.6 0.2 100.0 October 1972 5.0 14.7 39.9 41.9 0.1 100.0 September 1972 4 .9 12.7 31.2 53.3 100.0 August 1972 4.9 12.7 30.9 52.9 0.1 100.0 Unadjusted November 1972 5.6 16.6 34.1 43.6 0.2 100.0 October 1972 5.3 15.5 34.9 44.2 0.1 100.0 November 1971 5.5 15.7 30.1 48.8 0.1 100.0 Note: Details may not add to totals because of rounding and construction of seasonal Digitizedfactor for sFRASER by sector .