Research on Flavonoids Contents in Fructus Sophorae with Capillary Zone Electrophoresis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Addition of Clopidogrel to Aspirin in 45 852 Patients with Acute Myocardial Infarction: Randomised Placebo-Controlled Trial
Articles Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group* Summary Background Despite improvements in the emergency treatment of myocardial infarction (MI), early mortality and Lancet 2005; 366: 1607–21 morbidity remain high. The antiplatelet agent clopidogrel adds to the benefit of aspirin in acute coronary See Comment page 1587 syndromes without ST-segment elevation, but its effects in patients with ST-elevation MI were unclear. *Collaborators and participating hospitals listed at end of paper Methods 45 852 patients admitted to 1250 hospitals within 24 h of suspected acute MI onset were randomly Correspondence to: allocated clopidogrel 75 mg daily (n=22 961) or matching placebo (n=22 891) in addition to aspirin 162 mg daily. Dr Zhengming Chen, Clinical Trial 93% had ST-segment elevation or bundle branch block, and 7% had ST-segment depression. Treatment was to Service Unit and Epidemiological Studies Unit (CTSU), Richard Doll continue until discharge or up to 4 weeks in hospital (mean 15 days in survivors) and 93% of patients completed Building, Old Road Campus, it. The two prespecified co-primary outcomes were: (1) the composite of death, reinfarction, or stroke; and Oxford OX3 7LF, UK (2) death from any cause during the scheduled treatment period. Comparisons were by intention to treat, and [email protected] used the log-rank method. This trial is registered with ClinicalTrials.gov, number NCT00222573. or Dr Lixin Jiang, Fuwai Hospital, Findings Allocation to clopidogrel produced a highly significant 9% (95% CI 3–14) proportional reduction in death, Beijing 100037, P R China [email protected] reinfarction, or stroke (2121 [9·2%] clopidogrel vs 2310 [10·1%] placebo; p=0·002), corresponding to nine (SE 3) fewer events per 1000 patients treated for about 2 weeks. -

Annual Report 2000 Corporate Structure
CHARACTERISTICS OF THE GROWTH ENTERPRISE MARKET (“GEM”) OF THE STOCK EXCHANGE OF HONG KONG LIMITED (THE “EXCHANGE”) GEM has been established as a market designed to accommodate companies to which a high investment risk may be attached. In particular, companies list on GEM with neither track record of profitability nor any obligation forecast future profitability. Furthermore, there may be risks arising out of the emerging nature of companies listed on GEM and the business sectors or countries in which the companies operate. Prospective investors should be aware of the potential risks of investing in such companies and should make the decision to invest only after due and careful consideration. The greater risk profile and other characteristics of GEM mean that it is a market more suited to professional and other sophisticated investors. Given the emerging nature of companies listed on GEM, there is a risk that securities traded on GEM may be more susceptible to highmarket volatility than securities traded on the Main Board and no assurance is given that there will be a liquid market in the securities traded on GEM. The principal means of information dissemination on GEM is publication on the internet website operated by the Exchange. Listed companies are not generally required to issue paid announcements in gazetted newspapers. Accordingly, prospective investors should note that they need to have access to the GEM website in order to obtain up-to-date information on GEM-listed issuers. Contents Corporate Information 2 Corporate Structure -

Analysis of Spatial-Temporal Distribution of Notifiable Respiratory
Li et al. BMC Public Health (2021) 21:1597 https://doi.org/10.1186/s12889-021-11627-6 RESEARCH ARTICLE Open Access Analysis of spatial-temporal distribution of notifiable respiratory infectious diseases in Shandong Province, China during 2005– 2014 Xiaomei Li1†, Dongzhen Chen1,2†, Yan Zhang3†, Xiaojia Xue4, Shengyang Zhang5, Meng Chen6, Xuena Liu1* and Guoyong Ding1* Abstract Background: Little comprehensive information on overall epidemic trend of notifiable respiratory infectious diseases is available in Shandong Province, China. This study aimed to determine the spatiotemporal distribution and epidemic characteristics of notifiable respiratory infectious diseases. Methods: Time series was firstly performed to describe the temporal distribution feature of notifiable respiratory infectious diseases during 2005–2014 in Shandong Province. GIS Natural Breaks (Jenks) was applied to divide the average annual incidence of notifiable respiratory infectious diseases into five grades. Spatial empirical Bayesian smoothed risk maps and excess risk maps were further used to investigate spatial patterns of notifiable respiratory infectious diseases. Global and local Moran’s I statistics were used to measure the spatial autocorrelation. Spatial- temporal scanning was used to detect spatiotemporal clusters and identify high-risk locations. Results: A total of 537,506 cases of notifiable respiratory infectious diseases were reported in Shandong Province during 2005–2014. The morbidity of notifiable respiratory infectious diseases had obvious seasonality with high morbidity in winter and spring. Local Moran’s I analysis showed that there were 5, 23, 24, 4, 20, 8, 14, 10 and 7 high-risk counties determined for influenza A (H1N1), measles, tuberculosis, meningococcal meningitis, pertussis, scarlet fever, influenza, mumps and rubella, respectively. -

Download Article (PDF)
International Conference on Arts, Design and Contemporary Education (ICADCE 2015) Study on "Primitivity" of Folk Culture Take “Yi Gou Gou” as Example Lin Shen Li Tian School of Art Linyi County Federation of literary and Art Circles Shandong Women‘s University Linyi, Shandong, China, 250002 Jinan, Shandong, China, 250002 Abstract—When human makes reflections on the result of and is purposed to explore its aesthetic connotation in a form "cultural evolution", "primitivity" as a new concept of value, of folk opera and its value of "primitive" cultural heritage, so is becoming a hot topic of modern people. It has been a as to make better inheritance and development of national systematic project of the grand strategy of "developing a intangible cultural heritage such as the endangered local country by culture” to discover, protect and re-value the small opera. "primitive" folk culture. "Yi Gou Gou" is a small folk opera, popular in the Northwest region of Shandong Province, and has a history more than two hundred years. In 2006, it was II. THE INTANGIBLE CULTURAL HERITAGE: FOLK OPERA appointed as national nonmaterial cultural heritage. This "YI GOU GOU" paper made discussion on the origin and inheritance, types and "Yi Gou Gou" was appointed as "state-level intangible characteristics, regional influence and protection and cultural heritage" by the state council of the People's discovery of “Yi Gou Gou” based on its background of Republic of China and the ministry of culture in June 2006, regional history and culture, through interviewing the which is not occasional. inheritor of “Yi Gou Gou” and visiting the program rehearsal and discovering and arranging firsthand information. -

Strategic Development of Vegetable Supply Chains in Dezhou
Strategic development of vegetable supply chains in Dezhou Result of the fact finding mission from February 27 to March 2, 2017 Authors: Chris de Visser, Joost Snels, Eric Poot & Qiu Yu Tong Strategic development of vegetable supply chains in Dezhou Result of the fact finding mission from February 27 to March 2, 2017 Authors Chris de Visser, Joost Snels, Eric Poot & Qiu Yu Tong, Wageningen University & Research This study was carried out by Wageningen University & Research and was commissioned and financed by the Dezhou government Wageningen, February 2018 Report 756 Visser, C.L.M. de, Snels, J. & Poot, E., 2017. Strategic development of vegetable supply chains in Dezhou. Result of a fact finding mission from February 27 to March 2, 2017. Report 756 Keywords: vegetable supply chains, Dezhou, strategic development © 2018 Wageningen, Stichting Wageningen Research, P.O. Box 16, 6700 AA Wageningen, The Netherlands; T +31 (0)317 48 07 00; www.wur.nl Chamber of Commerce no. 09098104 te Arnhem VAT NL no. 8065.11.618.B01 The intellectual property rights of this report are owned by the Municipal Government of Dezhou City and Wageningen University & Research. The report is confidential. Stichting Wageningen Research is not liable for any adverse consequences resulting from the use of data from this publication. Wageningen University & Research, Confidential Report DOI: https://doi.org/10.18174/440955 Photo cover: Traditional Chinese solar greenhouse in Dezhou, © Chris de Visser Contents Contents 3 Preface 5 1 Introduction 7 1.1 General remarks 7 1.2 Basic description of the sector in Dezhou 7 1.3 Challenges 10 2 Ambition and goals of Dezhou City 13 2.1 Ambition 13 2.1.1 Fundamental principles 13 2.2 Main development goals 14 2.3 Specific development goals 14 2.3.1 Improve market supply. -

Emerging Development and Cooperation in Land Use Planning Along the Yellow River Under New Regionalism 47Th ISOCARP Congress 2011
Fang Wei Emerging Development and Cooperation in Land Use Planning along the Yellow River under New Regionalism 47th ISOCARP Congress 2011 Emerging Development and Cooperation in Land Use Planning along the Yellow River under New Regionalism: A Case Study by Jinan City, Shandong Province, China 1 INTRODUCTION Bearing some resemblance to North America in the early 1990s, China is currently issuing various regional developments and spatial planning strategies, aiming to coordinate development of various cities in regional context. In the final period of 11th Five Year Plan, almost 30 planning documents have been released in 2009 and 2010, which involved not only the traditional urban regions such as Lower Yangtze River Delta and Pearl River Delta, but also some less developed provinces in Middle and Western China (Jin, 2011). Among these initiatives, plans can be categorized into two kinds: one is of policy zone(Zhengce Qu) and the other is of comprehensive reform experimental zone (Zonghe Gaige Shiyan Qu) (Figure 1). Meanwhile, a serious of territorial plans has also been drafted such as national urban system planning, national main function plan, etc. The dynamic initiatives mentioned above demonstrated the great ambition from different departments of central state to inspire and ordinate newly developing local-level region. This trend has been accelerated by establishment of intra- and inter-regional mass transit infrastructure. High Speed Railway (HSR) between Beijing and Shanghai is the most significant case. It is expected such transit infrastructure will serve for compressing time-space among the cities and will in favor of tourism, tertiary industry and communication for future development. -

Prescribing Behaviour of Village Doctors Under China•S New
Social Science & Medicine 68 (2009) 1775–1779 Contents lists available at ScienceDirect Social Science & Medicine journal homepage: www.elsevier.com/locate/socscimed Prescribing behaviour of village doctors under China’s New Cooperative Medical Schemeq Xiaoyun Sun a,*, Sukhan Jackson b, Gordon A. Carmichael c, Adrian C. Sleigh c a Health Department of Shandong Province, Division of Maternal & Child Health and Community Health, 9 Yan Dong Xin Road, Jinan, Shandong 250014, China b School of Economics, The University of Queensland, St Lucia, Brisbane, Queensland 4072, Australia c National Centre for Epidemiology and Population Health, The Australian National University, Canberra, ACT 0200, Australia article info abstract Article history: In 2003, China introduced a new community-based rural health insurance called the New Cooperative Available online 1 April 2009 Medical Scheme (NCMS). In 2005, to assess the NCMS effects on village doctors’ prescribing behaviour, we compared an NCMS county and a non-NCMS county in Shandong Province. We collected information Keywords: from a representative total of 2271 patient visits in 30 village health stations (15 per county). China The average number of drugs prescribed (4.6 in the NCMS county vs. 3.1 in the non-NCMS county) and Village doctor use of antibiotics (72.4% vs. 59.3%) and injections (65.1% vs. 56.3%) were high in both counties, and higher New Cooperative Medical Scheme in the NCMS county. Within NCMS villages, prescribing for insured vs. uninsured patients showed Prescribing behaviour Antibiotics a similar pattern with more drugs, antibiotics and injections for those insured. Overall, for NCMS Health insurance patients, the prescription excess was about equal in value to their 20% fee discount. -

Response of Different Band Combinations in Gaofen-6 WFV for Estimating of Regional Maize Straw Resources Based on Random Forest Classification
sustainability Article Response of Different Band Combinations in Gaofen-6 WFV for Estimating of Regional Maize Straw Resources Based on Random Forest Classification Huawei Mou 1,2,3, Huan Li 1,2,4, Yuguang Zhou 1,5,6,7,* and Renjie Dong 1,2,4,5,8 1 Bioenergy and Environment Science & Technology Laboratory, College of Engineering, China Agricultural University, Beijing 100083, China; [email protected] (H.M.); [email protected] (H.L.); [email protected] (R.D.) 2 Key Laboratory of Clean Production and Utilization of Renewable Energy, Ministry of Agriculture and Rural Affairs, Beijing 100083, China 3 Department of Agricultural Systems Management, University of Missouri, Columbia, MO 65211, USA 4 National Center for International Research of BioEnergy Science and Technology, Ministry of Science and Technology, Beijing 100083, China 5 State R&D Center for Efficient Production and Comprehensive Utilization of Biobased Gaseous Fuels, National Energy Administration, Beijing 100083, China 6 National Energy R&D Center for Biomass, China Agricultural University, Beijing 100193, China 7 Prataculture Machinery and Equipment Research Center, College of Engineering, China Agricultural University, Beijing 100083, China 8 Yantai Institute, China Agricultural University, Yantai 264670, China * Correspondence: [email protected]; Tel.: +86-10-6273-7858 Abstract: Maize straw is a valuable renewable energy source. The rapid and accurate determination of its yield and spatial distribution can promote improved utilization. At present, traditional straw Citation: Mou, H.; Li, H.; Zhou, Y.; estimation methods primarily rely on statistical analysis that may be inaccurate. In this study, the Dong, R. Response of Different Band Gaofen 6 (GF-6) satellite, which combines high resolution and wide field of view (WFV) imaging Combinations in Gaofen-6 WFV for Estimating of Regional Maize Straw characteristics, was used as the information source, and the quantity of maize straw resources and Resources Based on Random Forest spatial distribution characteristics in Qihe County were analyzed. -

CHINA the Church of Almighty God: Prisoners Database (1663 Cases)
CHINA The Church of Almighty God: Prisoners Database (1663 cases) Prison term: 15 years HE Zhexun Date of birth: On 18th September 1963 Date and place of arrest: On 10th March 2009, in Xuchang City, Henan Province Charges: Disturbing social order and using a Xie Jiao organization to undermine law enforcement because of being an upper-level leader of The Church of Almighty God in mainland China, who was responsible for the overall work of the church Statement of the defendant: He disagreed with the decision and said what he believed in is not a Xie Jiao. Court decision: In February 2010, he was sentenced to 15 years in prison by the Zhongyuan District People’s Court of Zhengzhou City, Henan Province. Place of imprisonment: No. 1 Prison of Henan Province Other information: He was regarded by the Chinese authorities as a major criminal of the state and had long been on the wanted list. To arrest him, authorities offered 500,000 RMB as a reward to informers who gave tips leading to his arrest to police. He was arrested at the home of a Christian in Xuchang City, Henan Province. Based on the information from a Christian serving his sentence in the same prison, HE Zhexun was imprisoned in a separate area and not allowed to contact other prisoners. XIE Gao, ZOU Yuxiong, SONG Xinling and GAO Qinlin were arrested in succession alongside him and sentenced to prison terms ranging from 11 to 12 years. Source: https://goo.gl/aGkHBj Prison term: 14 years MENG Xiumei Age: Forty-one years old Date and place of arrest: On 14th August 2014, in Xinjiang Uyghur Autonomous Region Charges: Using a Xie Jiao organization to undermine law enforcement because of being a leader of The Church of Almighty God and organizing gatherings for Christians and the work of preaching the gospel in Ili prefecture Statement of the defendant: She claimed that her act did not constitute crimes. -

Engagement Or Control? the Impact of the Chinese Environmental Protection Bureaus’ Burgeoning Online Presence in Local Environmental Governance
This is a repository copy of Engagement or control? The impact of the Chinese environmental protection bureaus’ burgeoning online presence in local environmental governance. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/147591/ Version: Accepted Version Article: Goron, C and Bolsover, G orcid.org/0000-0003-2982-1032 (2020) Engagement or control? The impact of the Chinese environmental protection bureaus’ burgeoning online presence in local environmental governance. Journal of Environmental Planning and Management, 63 (1). pp. 87-108. ISSN 0964-0568 https://doi.org/10.1080/09640568.2019.1628716 © 2019 Newcastle University. This is an author produced version of an article published in Journal of Environmental Planning and Management. Uploaded in accordance with the publisher's self-archiving policy. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Engagement or control? The Impact of the Chinese Environmental Protection Bureaus’ Burgeoning Online Presence in Local Environmental Governance. -

Global Environment Facility (GEF) Operations
Innovative transformation of China?s food production systems and agroecological landscapes Part I: Project Information Name of Parent Program Food Systems, Land Use and Restoration (FOLUR) Impact Program GEF ID 10246 Project Type FSP Type of Trust Fund GET CBIT/NGI CBIT NGI Project Title Innovative transformation of China?s food production systems and agroecological landscapes Countries China Agency(ies) FAO, World Bank Other Executing Partner(s): Ministry of Agriculture and Rural Affairs (MARA), Hubei Province Department of Agriculture and Rural Affairs Executing Partner Type Government GEF Focal Area Multi Focal Area Taxonomy Chemicals and Waste, Focal Areas, Pesticides, Climate Change, Climate Change Mitigation, Agriculture, Forestry, and Other Land Use, Climate Change Adaptation, Climate resilience, Land Degradation, Sustainable Land Management, Sustainable Agriculture, Restoration and Rehabilitation of Degraded Lands, Biodiversity, Mainstreaming, Agriculture and agrobiodiversity, Influencing models, Demonstrate innovative approache, Transform policy and regulatory environments, Strengthen institutional capacity and decision-making, Stakeholders, Local Communities, Indigenous Peoples, Private Sector, SMEs, Large corporations, Beneficiaries, Type of Engagement, Consultation, Participation, Partnership, Civil Society, Academia, Gender Equality, Gender Mainstreaming, Sex-disaggregated indicators, Women groups, Participation and leadership, Gender results areas, Access to benefits and services, Integrated Programs, Food Systems, Land Use -
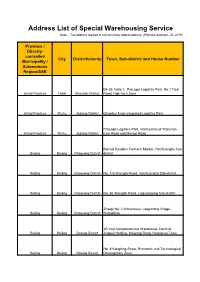
Address List of Special Warehousing Service Note: the Address Marked in Red Are Newly Added Address
Address List of Special Warehousing Service Note: The address marked in red are newly added address. (Effective date:Apr. 28, 2019) Province / Directly- controlled City District/county Town, Sub-district and House Number Municipality / Autonomous Region/SAR B4-25, Gate 1, ProLogis Logistics Park, No.1 Tiedi Anhui Province Hefei Shushan District Road, High-tech Zone Anhui Province Wuhu Jiujiang District Qingshui Ande Integrated Logistics Park ProLogis Logistics Park, Intersection of Wanchun Anhui Province Wuhu Jiujiang District East Road and Mengxi Road Behind Daludian Farmer's Market, Heizhuanghu Sub- Beijing Beijing Chaoyang District district Beijing Beijing Chaoyang District No. 1-9 Zhangtai Road, Heizhuanghu Sub-district Beijing Beijing Chaoyang District No. 66 Xiangbin Road, Laiguangying Sub-district Zhaopi No. 2 Warehouse, Laojuntang Village, Beijing Beijing Chaoyang District Shibalidian JD Yayi Comprehensive Warehouse, North of Beijing Beijing Daxing District Jingwei Holding, Weiyong Road, Huangcun Town No. 8 Kangding Street, Economic and Technological Beijing Beijing Daxing District Development Zone Beijing Beijing Daxing District No. 70 Weiyong Road Beijing Beijing Daxing District CEIEC, Xinghai 1st Street No. 11 Jinxiu Street, Yizhuang Economic and Technological Development Zone/No. 18 Yuncheng Street, Yizhuang Economic and Technological Beijing Beijing Daxing District Development Zone Beijing Beijing Daxing District No. 8 Kangding Street, Yizhuang Beijing Beijing Fengtai District No.102 Yangshuzhuang No. 3 Area F2D, Jinxiudadi