Composition for Optical Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Chemical Capabilities Listing Laboratory, R&D, Industrial and Manufacturing Applications
Page 1 of 3 Chemical Capabilities Listing Laboratory, R&D, Industrial and Manufacturing Applications Acacia, Gum Arabic Barium Oxide Chromium Trioxide Acetaldehyde Bentonite, White Citric Acid, Anhydrous Acetamide Benzaldehyde Citric Acid, Monohydrate Acetanilide Benzoic Acid Cobalt Oxide Acetic Acid Benzoyl Chloride Cobaltous Acetate Acetic Anhydride Benzyl Alcohol Cobaltous Carbonate Acetone Bismuth Chloride Cobaltous Chloride Acetonitrile Bismuth Nitrate Cobaltous Nitrate Acetyl Chloride Bismuth Trioxide Cobaltous Sulfate Aluminium Ammonium Sulfate Boric Acid Cottonseed Oil Aluminon Boric Anhydride Cupferron Aluminum Chloride, Anhydrous Brucine Sulfate Cupric Acetate Aluminum Chloride, Hexahydrate n-Butyl Acetate Cupric Bromide Aluminum Fluoride n-Butyl Alcohol Cupric Carbonate, Basic Aluminum Hydroxide tert-Butyl Alcohol Cupric Chloride Aluminum Nitrate Butyric Acid Cupric Nitrate Aluminum Oxide Cadmium Acetate Cupric Oxide Aluminum Potassium Sulfate Cadmium Carbonate Cupric Sulfate, Anhydrous Aluminum Sulfate Cadmium Chloride, Anhydrous Cupric Sulfate, Pentahydrate 1-Amino-2-Naphthol-4-Sulfonic Acid Cadmium Chloride, Hemipentahydrate Cuprous Chloride Ammonium Acetate Cadmium Iodide Cuprous Oxide, Red Ammonium Bicarbonate Cadmium Nitrate Cyclohexane Ammonium Bifluoride Cadmium Oxide Cyclohexanol Ammonium Bisulfate Cadmium Sulfate, Anhydrous Cyclohexanone Ammonium Bromide Cadmium Sulfate, Hydrate Devarda's Alloy Ammonium Carbonate Calcium Acetate Dextrose, Anhydrous Ammonium Chloride Calcium Carbide Diacetone Alcohol Ammonium Citrate -

Llernents F;--L.- -+[;;Ffi;L
7 L,, Science 10 Review for Chemistry Test I Metalloids. Metals and Nonmetals THE Llernents F;--l.- -+[;;ffi;l f nlk:lr fietls I *hEr l,tett. fl.] Ilka:lne Einn idetats I tletlotas f-1 lrar$tron Melrts f] crhe. tionmelals I Lan!fiatrtdes I uotoge"u I a:r,iro:. ! rrcrrec::r: l9 Os lr Pt Au Hg II bE 'llir ' Pu llnm llcm br. r,,-- ll,*"* llL.* r...-.llr,r'... Pbs.org.lnova,'ect'uution Identify each of the following elements as a metailoid, metal or nonmetal. a) iron rn'.*t-( b) carbon h uv' ,n"*^l c) neon no^^c*a- I d) tungsten YV\C+LI e) astatine hon *..*-( f) antimony rvrr '|atlo ; J g) aluminum hr\o {-L { h) terbium r.-e * ,- I \ \ Tvoes of Ions 5l 1. monatomic ions (MI) ion) -> 1 type of atom involved:Nal* (sodium Cl1- (chloride ion) 2. polyatomic ions (PI) NH+l* -> more than 1 tlpe of atom involved: NO31-, BO:3-' -> end in: "ate" iel sulfate ion, SO+2- "ite" iel chlorite ion, ClOzl- ion (o22.) ..ide,, cyanide ion (CNl), hydroxide ion (oHl-), peroxide a ions of multivalent metals QMM) possible ion: Cu1*, Cu2* .> 1 type of atom involved but more than one (I) ion, copper (II) ion -) names include roman numerals: coppff Identify each of the follorving ions as MI, PI or IMM' u) gg' 9T b) Znz+ {^c c) aluminum ion 4 t" lAt avA d) vr* V"'l1nr )- O -:- e) sulfite ion 5 u, l)- lrM fl lead (IV) ion /) -- g) ammonium ion ,Vt{.} rL - oi- N h) thiocyanate ion 5r t)- i) Lu3n lAT l j) PzOza- ff k) oxide ion AT Ionic Compounds 1. -
TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia
United States Office Pollution EPA 745-R-00-005 Environmental Protection Prevention and Toxics Revised April 2018 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia Section 313 of the Emergency Planning and Community Right-to-Know Act of 1986 (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. EPCRA section 313 is also known as the Toxics Release Inventory (TRI). CONTENTS Section 1. Introduction ............................................................................................................ 3 1.1 Who Must Report ...................................................................................... 3 1.2 Thresholds ................................................................................................. 3 1.3 Chemical Sources of Aqueous Ammonia ................................................. 4 1.4 De Minimis Concentrations ...................................................................... -

2010 AIGCC-Meeting-Summary-And-Recommendations-Report 0.Pdf
U.S. DEPARTMENT OF ENERGY NATIONAL ENERGY TECHNOLOGY LABORATORY FINAL REPORT STRATEGIC CENTER FOR COAL ADVANCED INTEGRATED GASIFICATION COMBINED CYCLE FY 2010 PEER REVIEW MEETING Pittsburgh, Pennsylvania December 7–11, 2009 MEETING SUMMARY AND RECOMMENDATIONS REPORT José D. Figueroa NETL Project Manager and Peer Review Coordinator (412) 386-4966 Meeting Host Organizations Technology & Management Services, Inc. Steven T. Ostheim (412) 347-2322 Leonardo Technologies, Inc. Massood Ramezan (412) 386-6451 Review Panel AMERICAN SOCIETY OF MECHANICAL ENGINEERS Daniel J. Kubek, Chair, Peer Review Panel Richard Laudenat, Chair, Peer Review Executive Committee Michael Tinkleman, Director, Research ASME Center for Research and Technology Development (202) 785-7394 Meeting Facilitator and Final Report Ross Brindle, Energetics Incorporated (410) 953-6239 Work Done Under Prime Contracts Number DE-AC26-05NT41816 (Subtask 300.01.04) and DE-FE0004002 (Subtask 300.01.05) DISCLAIMER This report was prepared through the collaborative efforts of The American Society of Mechanical Engineers (ASME) Center for Research and Technology Development (hereinafter referred to as the Society or ASME) and sponsoring companies. Neither the Society, nor the sponsors, nor the Society’s subcontractors, nor any others involved in the preparation or review of this report, nor any of their respective employees, members or other persons acting on their behalf, make any warranty, expressed or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed or referred to in this report, or represent that any use thereof would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Society, the sponsors, or others involved in the preparation or review of this report, or agency thereof. -

Chapter 7 - PHYSICAL and CHEMICAL ANALYSES
Water Quality Monitoring - A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes Edited by Jamie Bartram and Richard Ballance Published on behalf of United Nations Environment Programme and the World Health Organization © 1996 UNEP/WHO ISBN 0 419 22320 7 (Hbk) 0 419 21730 4 (Pbk) Chapter 7 - PHYSICAL AND CHEMICAL ANALYSES This chapter was prepared by R. Ballance In compiling this chapter, care has been taken to avoid procedures that require delicate or sophisticated equipment. For many of the variables for which methods of analysis are presented here, further information relating to their selection and inclusion in water quality monitoring and assessment programmes (such as their environmental significance, normal ranges of concentrations, and behaviour in the aquatic environment) can be found in the companion guidebook Water Quality Assessments. 7.1 Preparation and use of chemical reagents The following general rules should be followed in the preparation and use of chemical reagents. The best quality chemical reagents available should be used - normally “analytical reagent grade”. For most laboratory purposes, water distilled in a borosilicate glass still or a tin still will be satisfactory. For preparing some reagents, dilution water requires special treatment, such as a second distillation, boiling to drive off CO2 or passing through a mixed bed ion exchanger. Where such special treatment is necessary, this is stated. Recipes for the preparation of reagents usually give directions for the preparation of a 1-litre volume. For those reagents that are not used often, smaller volumes should be prepared by mixing proportionally smaller quantities than those given in the recipe. -

Substrate Patent Application Publication Feb
US 2013 0037111A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0037111A1 Mitzi et al. (43) Pub. Date: Feb. 14, 2013 (54) PROCESS FOR PREPARATION OF HOIL 3L/8 (2006.01) ELEMENTAL CHALCOGEN SOLUTIONS C09D II/00 (2006.01) AND METHOD OF EMPLOYING SAD B82Y-3O/OO (2011.01) SOLUTIONS IN PREPARATION OF (52) U.S. Cl. ... 136/264; 438/95; 106/31.13; 252/182,32: KESTERITE FILMS 257/E31.027; 977/773 (75) Inventors: David Brian Mitzi, Mahopac, NY (US); (57) ABSTRACT Xiaofeng Qiu, Sleepy Hollow, NY (US) Techniques for preparing chalcogen-containing solutions (73) Assignee: International Business Machines using an environmentally benign borane-based reducing Corporation, Armonk, NY (US) agent and solvents under ambient conditions, as well as appli cation of these solutions in a liquid-based method for depo (21) Appl. No.: 13/207,248 sition of inorganic films having copper (Cu), Zinc (Zn), tin (Sn), and at least one of sulfur (S) and selenium (Se) are (22) Filed: Aug. 10, 2011 provided. In one aspect, a method for preparing a chalcogen containing solution is provided. The method includes the Publication Classification following steps. At least one chalcogen element, a reducing agent and a liquid medium are contacted under conditions (51) Int. C. Sufficient to produce a homogenous Solution. The reducing HOIL 31/032 (2006.01) agent (i) contains both boron and hydrogen, (ii) is substan C09K3/00 (2006.01) tially carbon free and (iii) is substantially metal free. 404 402 302 204 202 Substrate Patent Application Publication Feb. 14, 2013 Sheet 1 of 5 US 2013/00371.11 A1 100 Contact metal source(s) with the elemental chalcogen solution under conditions sufficient to form metal-chalcogen nanoparticles containing Cu, Sn, Zn and at least 102 one of S and Se. -

United States Patent Office Patented Sept
2,851,399 United States Patent Office Patented Sept. 9, 1958 A. fective stabilizers, they are comparatively easy to handle, 2,851,399 and they avoid the hazards involved in the use of volatile, STABELIZED PATINUM-ALUMNA CATALYSS highly toxic substances such as hydrogen selenide. (COTANANG SELENEJM in another aspect of our invention, a platinum-alumina 5 composite is exposed to contact with a selenium-contain Harry vi. Brethaia, Haganaoad, Ead., and Edisassid Field, ing substance in a sufficient quantity to incorporate a Chicago, S., assignors to Standard Oil Congary, Cai. stabilizing quantity of selenium therein, as defined above. cago, A., a corporation of Indiana This technique is effective broadly for the treatment of No 5)rawing. Application Jane 29, 1955 the prior-art platinum-alumina catalysts. Seria No. 518,926 O The stabilization of catalysts with selenium in accord 16 Claims. (C. 96-50) ofance ways. with our invention can be carried out in a variety According to one technique, a platinum-alumina com This invention relates to the conversion of hydrocar posite, prepared by any of the methods known in the art, bons. More particularly, it relates to the hydroforming can be impregnated with a selenium-containing solution. of petroleum naphthas over alumina-supported platinum The composite should thereafter be dried at 200 to 600 catalysts, and to stabilized catalysts for such processes. F. for 6 to 24 hours, then calcined at 800 to 1200° F. for The treatment of petroleum hydrocarbons for various 1 to 24 hours. Aqueous solutions are generally preferred purposes is now largely carried out by catalytic means. -

Emergency Planning and Community Right-To-Know Act Section 313 Reporting Guidance for Semiconductor Manufacturing
TABLE OF CONTENTS Page ACKNOWLEDGMENT ..................................................... vi OVERVIEW ..............................................................vii CHAPTER 1 - INTRODUCTION ............................................. 1-1 1.0 PURPOSE .......................................................... 1-1 1.1 Background on EPCRA Section 313 and PPA Section 6607 .............. 1-2 CHAPTER 2 - REPORTING REQUIREMENTS ................................. 2-1 2.0 PURPOSE .......................................................... 2-1 2.1 Must You Report? ............................................. 2-2 2.2 SIC Code Determination ........................................ 2-4 2.3 Number of Employees .......................................... 2-6 2.4 Manufacturing, Processing, and Otherwise Use of EPCRA Section 313 Chemicals or Chemical Categories ................................. 2-7 2.5 Activity Categories ............................................. 2-8 2.6 How Do You Report? ......................................... 2-10 2.7 Form R ..................................................... 2-11 2.8 Alternate Threshold and Form A .................................. 2-12 2.9 Trade Secrets ................................................ 2-13 2.10 Recordkeeping ............................................... 2-14 CHAPTER 3 - EPCRA SECTION 313 CHEMICAL OR CHEMICAL CATEGORY ACTIVITY THRESHOLD DETERMINATIONS ................................. 3-1 3.0 PURPOSE .......................................................... 3-1 3.1 Step 1 - Identify -

TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia
United States Environmental Office of Pollution Prevention and Toxics EPA 745-B-19-002 Protection Agency Washington, DC 20460 Revised February 2019 TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia Section 313 of the Emergency Planning and Community Right-to-Know Act of 1986 (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report the annual quantity of such chemicals entering each environmental medium. Such facilities must also report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. EPCRA section 313 is also known as the Toxics Release Inventory (TRI). CONTENTS INTRODUCTION .............................................................................................................. 1 Section 1.1 Chemical Sources of Aqueous Ammonia .............................................................................. 1 Section 1.2 De Minimis Concentrations ................................................................................................... 1 Section 1.3 General TRI Reporting Instructions ....................................................................................... 1 GUIDANCE FOR REPORTING AQUEOUS AMMONIA ........................................... 2 Section 2.1 Determining Threshold and Release Quantities for Ammonia .............................................. 2 Section 2.2 Chemical Sources of Aqueous Ammonia ............................................................................. -
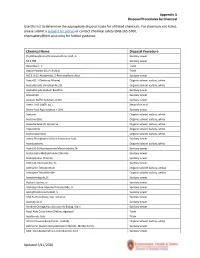
Appendix a Disposal Procedures by Chemical Updated 5/11/2020 Use
Appendix A Disposal Procedures by Chemical Use this list to determine the appropriate disposal route for all listed chemicals. For chemicals not listed, please submit a request for pickup or contact chemical safety (608-265-5700, [email protected]) for further guidance. Chemical Name Disposal Procedure (Cyclohexylamino)Propanesulfonic Acid, 3- Sanitary sewer 50 X TBE Sanitary sewer Absorbosil - 1 Trash Acacia Powder (Gum Arabic) Trash ACES, N-(2-Acetamido)-2-Aminosulfonic Acid Sanitary sewer Acetal (1,1-Diethoxy Ethane) Organic solvent carboy, white Acetaldehyde Dimethyl Acetal Organic solvent carboy, white Acetaldehyde Sodium Bisulfite Sanitary sewer Acetamide Sanitary sewer Acetate Buffer Solution, 0.2M Sanitary sewer Acetic Acid (<80% aq.) Neutralize acid Acetic Acid Aqu.Solution < 30% Sanitary sewer Acetone Organic solvent carboy, white Acetone (D6) Organic solvent carboy, white Acetone Ketal Of Glycerine Organic solvent carboy, white Acetonitrile Organic solvent carboy, white Acetonylacetone Organic solvent carboy, white Acetyl Phosphate (Lithium Potassium Salt) Sanitary sewer Acetylacetone Organic solvent carboy, white Acetyl-B-D-Mannosamine Monohydrate, N- Sanitary sewer Acetyl-beta-Methylcholine Chloride Sanitary sewer Acetylcholine Chloride Sanitary sewer Acetyl-D-Glucosamine, N- Sanitary sewer Acetylene Tetrabromide Organic solvent carboy, yellow Acetylene Tetrachloride Organic solvent carboy, yellow Acetylimidazole, N- Sanitary sewer Acetyl-L-Serine, o- Sanitary sewer Acetylpyridine Adenine Dinucleotide, 3- Sanitary sewer -

(12) United States Patent (10) Patent No.: US 9,150,694 B2 Aoki Et Al
US009 150694B2 (12) United States Patent (10) Patent No.: US 9,150,694 B2 Aoki et al. (45) Date of Patent: Oct. 6, 2015 (54) COMPOSITION FOR USE IN OPTICAL (56) References Cited MATERAL WITH HIGH REFRACTIVE INDEX AND HIGH STRENGTH U.S. PATENT DOCUMENTS (75) Inventors: TakashiO Aoki,O Osaka Prefecture (JP): 20092004/0254258 OO18308 A1 12/20041/2009 RNAHorikoshi ety al. Hirohito Ishizuka, Chiba Prefecture 2010.0004421 A1 1/2010 Horikoshi et al. .............. 528/59 (JP); Motoharu Takeuchi, Chiba 2010/0331515 A1 12/2010 Takeuchi et al. Prefecture (JP) FOREIGN PATENT DOCUMENTS (73) Assignee: MITSUBISHIGAS CHEMICAL COMPANY, INC., Tokyo (JP) JP 9-1 10979 4f1997 JP 2001-2783 1, 2001 (*) Notice: Subject to any disclaimer, the term of this E. 3:) 858 patent is extended or adjusted under 35 JP 2006-348.285 12/2006 U.S.C. 154(b) by 0 days. JP 2007-93862 4/2007 JP 2007-093862 4/2007 (21) Appl. No.: 13/319,857 JP 2007-238796 9, 2007 JP 2008-101,190 5, 2008 WO WO 2008/035643 A1 * 3, 2008 (22) PCT Filed: May 10, 2010 WO 2008. 136401 11, 2008 WO 2010/0736.13 T 2010 (86). PCT No.: PCT/UP2010/057909 S371 (c)(1) OTHER PUBLICATIONS C s (2), (4) Date: Feb. 27, 2012 Search report from International Application No. PCT/JP2010, 057909, mail date is Jul. 27, 2010. (87) PCT Pub. No.: WO2010/131631 Japan Office action, mail date is Jul. 1, 2014. PCT Pub. Date: Nov. 18, 2010 * cited by examiner (65) Prior Publication Data Primary Examiner — Susannah Chung US 2012/O142889 A1 Jun.