Sampling Techniques and Population Estimation for Athergona Reversura
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JTI Volume 2 Issue 4 Back Matter
INSECT SCIENCE AN D ITS APPLICATION The International Journal of Tropical Insect Science Editor: Thomas R Odhiambo International Centre of Insect Physiology and Ecology (ICIPE) List of Contents, Author and Subject Index for Volume 2,1981 PERGAMON PRESS OXFORD • NEW YORK • TORONTO SYDNEY • PARIS • FRANKFURT Downloaded from https://www.cambridge.org/core. IP address: 170.106.33.14, on 29 Sep 2021 at 15:28:11, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. https://doi.org/10.1017/S174275840000103X Insect Science and its Application The International Journal of Tropical Insect Science Sponsored by the International Centre of Insect Physiology and Ecology (ICIPE) and the African Association of Insect Scientists (AAIS) Editor-in-Chief Thomas R. Odhiambo International Centre of Insect Physiology and Ecology (ICIPE), P.O. Box 30772, Nairobi, Kenya Editorial Advisory Board F. T. Abu Shama, Khartoum, R. Levins, Boston, U.S.A. K. N. Saxena, Delhi, India Sudan M. Locke, Ontario, Canada H. Schmutterer, Giessen, P. L. Adkisson, Texas, U.S.A. F. G. Maxwell, Florida, U.S.A. F.R. Germany J. C. M. Carvalho, Rio de Janeiro, J. Meinwald, New York, U.S.A. L. M. Schoonhoven, Wageningen, Brazil P. L. Miller, Oxford, England The Netherlands R. Galun, Jerusalem, Israel J. Mouchet, Bondy, France J. E. Treherne, Cambridge, England T. Hidaka, Kyoto, Japan A. S. Msangi, Dar-es-Salaam, D. F. Waterhouse, Canberra, H. Hirumi, Nairobi, Kenya Tanzania Australia V. Landa, Prague, Czechoslovakia M. D. Pathak, Manila, A. Youdeowei, Ibadan, Nigeria The Philippines PUBLISHED QUARTERLY Publishing, Subscription and Advertising Offices: Headington Hill Hall, Oxford OX3 OBW, U.K. -

Products Against Shoot Fly, Atherigona Soccata
Journal of Entomology and Zoology Studies 2017; 5(3): 474-477 E-ISSN: 2320-7078 P-ISSN: 2349-6800 JEZS 2017; 5(3): 474-477 Efficacy of some novel insecticides and bio- © 2017 JEZS products against shoot fly, Atherigona Soccata Received: 10-03-2017 Accepted: 11-04-2017 (Rondani) in maize Sudhir Kumar Department of Entomology, Sardar Vallabhbhi Patel Sudhir Kumar, DV Singh, Kantipudi Rajesh Kumar and Awaneesh University of Agriculture & Kumar Technology, Meerut, Uttar Pradesh, India Abstract DV Singh An investigation to study the efficacy of some novel insecticides and bio-products against shoot fly, Department of Entomology, Atherigona soccata (Rondani) in maize was carried out for two consecutive seasons i.e. 2013 and 2014, Sardar Vallabhbhi Patel at Crop Research Centre, Sardar Vallabhbhai Patel University of Agriculture & Technology, Meerut. University of Agriculture & Efficacy of different insecticides and bio-products viz. Imidacloprid 70WS, Thiamethoxam 70WS, Technology, Meerut, Carbofuran 3G, Phorate 10G, NSKE, Cow urine-NSKE, Imidacloprid 70WS-NSKE and Thiamethoxam Uttar Pradesh, India 70WS-NSKE were evaluated against number of eggs of A. soccata revealed that the treatments, Imidacloprid 70WS-NSKE was found best to reduce the number of eggs laid by A. soccata i.e. 0.00 Kantipudi Rajesh Kumar Department of Entomology, eggs/plant (11 DAE) and 0.45 eggs/plant (22 DAE) followed by Thiamethoxam 70WS-NSKE with 0.45 Banaras Hindu University, eggs/plant (11 DAE) and 0.72 eggs/plant (22 DAE). However, the maximum number of eggs survival of Varanasi, Uttar Pradesh, India A. soccata was recorded with untreated control 1.85 eggs/plant (11 DAE) and 2.30 eggs/plant (22 DAE). -

What We Have Learned About the Bermudagrass Stem Maggot
WHAT WE HAVE LEARNED ABOUT THE BERMUDAGRASS STEM MAGGOT D.W. Hancock, W.G. Hudson, L.L. Baxter, and J.T. McCullers1 Abstract Since first being discovered in southern Georgia in July 2010, the bermudagrass stem maggot (BSM; Atherigona reversura Villeneuve) has infested and damaged forage bermudagrass (Cynodon dactylon) throughout the southeastern United States. Our objectives for this presentation were to summarize the available literature on this new, invasive species and provide additional insight from what is currently known about other Atherigona spp. The BSM, along with other Atherigona spp., are small, muscid flies native to Central and Southeast Asia. The adult fly of the BSM lays its eggs on bermudagrass leaves. Upon hatching, the BSM larva slips into the sheath, down the tiller, and penetrates the pseudostem at the first node. The BSM larva then feeds on the vascular tissue, sap, and (potentially) the subsequent decaying plant material before exiting the tiller, pupating in the soil, and emerging as a fly. As a result of the larval feeding, bermudagrass exhibits senescence and necrosis of the terminal leaves on the affected shoots. The affected leaves are easily pulled out of the sheath and show obvious damage near the affected node. In severe infestations, over 80% of the tillers in a given area may be affected. There is a paucity of information about the lifecycle of A. reversura and how it can be managed or controlled, but some information is available on basic larva behavior, fly physiology, and the potential differences in resistance among some bermudagrass varieties. Additional research is underway to better understand the lifecycle of this species, confirm and quantify the degree of preference A. -

Comparative Biology of Shoot Fly, Atherigona Soccata (Rondani) On
Chemical Science Review and Letters ISSN 2278-6783 Research Article Comparative Biology of Shoot Fly, Atherigona Soccata (Rondani) on Susceptible, Resistant Genotypes and Its F1 Hybrid of Sorghum S. Sekar1*, S. Jeyarani2 and N. Kumaravadivel3 1Ph.d Scholar, Department of Agricultural Entomology, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India 2Professor, Agricultural Entomology, Directorate of Open Distance Learning, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India 3Professor and Head, Department of Molecular Breeding and Bioinformatics, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India Abstract Comparative biology of Atherigona soccata (Rondani) was studied on Keywords: Shoot fly, biology, susceptible (K8), resistant (IS 2205) sorghum genotypes and its F1 hybrid. IS 2205 (Resistant genotype), Significant differences were observed between resistant susceptible genotypes K8 (Susceptible genotype) and its F1 hybrid with regard to larval, pupal and adult developmental parameters. Developmental periods of egg, larva and pupa was maximum on *Correspondence IS 2205, F1 hybrid and minimum on K8. Total life cycle of shoot fly was Author: S. Sekar prolonged on resistant genotype, IS 2205 (24.98 ±1.87 days) and its F1 hybrid Email: [email protected] (22.04 ±1.67 days) than susceptible genotype, K 8 (20.1±105 days). Length and width of egg, larva, pupa and growth indices like pupation (%), adult emergence (%), fecundity (nos./ female) ovipositional period (days) and adult longevity (male and female) were minimum on IS 2205 indicating higher degree of antibiosis in the resistant genotype. Observation on female: male ratio showed no significant variation between IS2205, F1 hybrid and K 8. Introduction Sorghum is an important food and fodder crop in India. -

Species of Atherigona in Andhra Pradesh
5)PANS 2314): 379 383 Crown copyright, 1977 Species of Atherigona in Andhra Pradesh K. V. Seshu Reddy and J. C. Davies ICRISA T, 1- 11-256 Begurnper, Hyderabad, India. Summary. Records of Arhengona spp, bred from cultlvdted cereals. mtnor m~lletsand wild grass hosts ere glven. A Iota1 01 19 plant hosts of Arherrgona specter IS Ilrted. A. soccata, sorghum shootfly, was by far the commonest spocles present on sorghum and ~t was alw r~ordedfrom malze, pearl m~llet,Echinochloa colonurn. Errochlos procera, Cyrnbopogon sp, and Paspalurn scrobicularum. Numbers of A. roccara bred from grasses were very low. Data showed that specles of Arhe~gonawere In general highly specific In choice of plant host A. lalcafa wds the commonest qrass l~vlng species, but tended to favour Echinochlm colonurn and E. crusgsll~.The dom~nant specter on pearl millet was A. appmximara, on Panicum ps~lopodium,A. pulla and on Digiraria adscendens, A. oryzae. An lnlerertlng dnd important record was the recovery of A. eriochloae from both sorghum and Er~ochloaprocara. Th~srprclcs was prev~ouslyonly known from the peratype descr~bedIn 1926. Other specles were bred from a range of host grasses. Introduction The sorghum shootfly, Atherigonasoccaa Rond., is well known as a serlous pest of sorghum in lndia (Jotwani eral., 1970). It is also a widely distributed and occas~onallydamaglng pest in Africa (Swa~neand Wyatt, 1954; Langham, 1968; Deeming, 1971). Control of the fly has been achieved uslng systemlc ~nsecticides(Jotwani and Sukhanl, 1968; Thobbietal., 1968; Barry, 1972). There have. however, been several reports of only part~alsuccess in controlling the fly with contact insect~cides(Swalne and Wyatt, 1954; Wheatley, 1961) or failure to control tt (Ingram, 1959). -

Nomenclatural Studies Toward a World List of Diptera Genus-Group Names
Nomenclatural studies toward a world list of Diptera genus-group names. Part V Pierre-Justin-Marie Macquart Evenhuis, Neal L.; Pape, Thomas; Pont, Adrian C. DOI: 10.11646/zootaxa.4172.1.1 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Evenhuis, N. L., Pape, T., & Pont, A. C. (2016). Nomenclatural studies toward a world list of Diptera genus- group names. Part V: Pierre-Justin-Marie Macquart. Magnolia Press. Zootaxa Vol. 4172 No. 1 https://doi.org/10.11646/zootaxa.4172.1.1 Download date: 02. Oct. 2021 Zootaxa 4172 (1): 001–211 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4172.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:22128906-32FA-4A80-85D6-10F114E81A7B ZOOTAXA 4172 Nomenclatural Studies Toward a World List of Diptera Genus-Group Names. Part V: Pierre-Justin-Marie Macquart NEAL L. EVENHUIS1, THOMAS PAPE2 & ADRIAN C. PONT3 1 J. Linsley Gressitt Center for Entomological Research, Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii 96817-2704, USA. E-mail: [email protected] 2 Natural History Museum of Denmark, Universitetsparken 15, 2100 Copenhagen, Denmark. E-mail: [email protected] 3Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PW, UK. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Whitmore: 15 Aug. 2016; published: 30 Sept. 2016 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 NEAL L. -

ENV /JM /M on O(2016)27 Unclassified
Unclassified ENV/JM/MONO(2016)27 Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 29-Jun-2016 ___________________________________________________________________________________________ _____________ English - Or. English ENVIRONMENT DIRECTORATE JOINT MEETING OF THE CHEMICALS COMMITTEE AND Unclassified ENV/JM/MONO(2016)27 THE WORKING PARTY ON CHEMICALS, PESTICIDES AND BIOTECHNOLOGY Cancels & replaces the same document of 29 June 2016 CONSENSUS DOCUMENT ON THE BIOLOGY OF SORGHUM (Sorghum bicolor (L.) Moench) Series on Harmonisation of Regulatory Oversight in Biotechnology No. 62 English JT03398806 Complete document available on OLIS in its original format - This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of Or. English international frontiers and boundaries and to the name of any territory, city or area. ENV/JM/MONO(2016)27 2 ENV/JM/MONO(2016)27 OECD Environment, Health and Safety Publications Series on Harmonisation of Regulatory Oversight in Biotechnology No. 62 Consensus Document on the Biology of Sorghum (Sorghum bicolor (L.) Moench) Environment Directorate Organisation for Economic Co-operation and Development Paris 2016 3 ENV/JM/MONO(2016)27 Also published in the Series on Harmonisation of Regulatory Oversight in Biotechnology: No. 1, Commercialisation of Agricultural Products Derived through Modern Biotechnology: Survey Results (1995) No. 2, Analysis of Information Elements Used in the Assessment of Certain Products of Modern Biotechnology (1995) No. 3, Report of the OECD Workshop on the Commercialisation of Agricultural Products Derived through Modern Biotechnology (1995) No. 4, Industrial Products of Modern Biotechnology Intended for Release to the Environment: The Proceedings of the Fribourg Workshop (1996) No. -
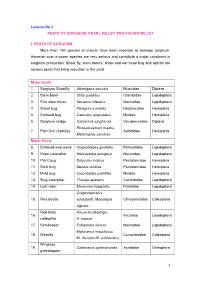
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

TEF: Post-Harvest Operations
TEF Post-harvest Operations - Post-harvest Compendium TEF: Post-harvest Operations Organization: Institute of Agricultural Research Organization, Holetta Agricultural Research Center (IARO) Author: Alemayehu Refera Edited by AGSI/FAO: Danilo Mejia (Technical), Beverly Lewis (Language & Style) Last reviewed: 14/05/2001 Contents Preface ................................................................................................................................ 2 1. Introduction ........................................................................................................................ 2 1.1 Economic and Social Impact of Tef ............................................................................. 7 1.2 World Trade ................................................................................................................. 9 1.3 Primary Product .......................................................................................................... 10 1.4 Secondary & Derived Products .................................................................................. 10 1.5 Consumer Preference ................................................................................................. 28 2. Post-Production Operations .............................................................................................. 30 2.1 Harvesting .................................................................................................................. 30 2.2 Threshing ................................................................................................................... -

Atherigona Soccata, Rondani L
AL SC R IEN TU C A E N F D O N U A N D D Journal of Applied and Natural Science 7 (1) : 77 – 82 (2015) A E I T L JANS I O P N P A ANSF 2008 Statistical relationship between date of sowing and the sorghum shootfly (Atherigona Soccata , Rondani L) S. T. Pavan Kumar *, Y. N. Havaldar 1, Shekharappa 2 and Adam Kamei 3 * Department of Agricultural Statistics, B. C. K. V., Mohanpur-741252 (West Bengal), INDIA 1Department of Agricultural Statistics, University of Agricultural Sciences, Dharwad-580005 (Karnataka), INDIA 2All India Coordinated Sorghum Crop Improvement Project, Main Agricultural Research Station, University of Agricultural Sciences, Dharwad-580005 (Karnataka), INDIA 3Department of Plant Pathology, B. C. K. V., Mohanpur-741252 ( West Bengal), INDIA *Corresponding author. E-mail : [email protected] Received: November 19, 2014; Revised received: February 16, 2015; Accepted: February 27, 2015 Abstract : The present study was based on the available data of eleven years for shoot fly from 2000-2010 for kharif season. Different models viz., linear and non linear were tried to fit, Amongst, the linear, quadratic and cubic models produced better coefficient of determination and the models viz., EGG(Shoot fly Egg) =3.760+0.196(DOS) (R 2=0.892) and EGG(Shoot fly Egg) =1.077+1.195(DOS)-0.087(DOS^2), which produced highest R 2 (0.896 at p=0.05) with less standard error (0.419) and quadratic model was also the best fit model in determining the oviposition of shoot fly, which explained 89.6 per cent variation in the oviposition of shoot fly for the 7 days after emergence of the sorghum crop. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -

Criteria for Selection of IPM Priorities Priority Listing
These priorities resulted from the March 16, 2016 meeting of the SERA 003 members including the IPM coordinators from the southern region and invited members of other groups such as the IPM centers. The priorities are a consensus document of the SERA 003 IPM group Criteria for Selection of IPM Priorities 1. Strong stakeholder identified need 2. Addresses economic, environmental and/or human health issues 3. Priority is relevant in two or more states or territories in the Southern Region Priority Listing Projects that address critical IPM issues resulting from ● Changes in management systems, ● Pesticide resistance (chemical or GMO) ● Invasive pests ● Loss of management tools ● Environmental changes Priority areas include, but are not limited to: ● New, emerging, and important pests / issues – domestic or introduced invasive pests INSECTS: ○ Brown marmorated stink bug (Halyomorpha halys) ○ Spotted wing drosophila (Drosophila suzukii) ○ Kudzu bug (Megacopta cribraria) ○ Sugarcane aphid (Melanaphis sacchari) ○ Sugarcane orange rust (Puccinia kuehnii) ○ Tropical fruit flies (Oriental fruit fly (Bactrocera dorsalis), Mediterranean fruit fly (Ceratitis capitata), Mexican fruit fly (Anastrepha ludens)) ○ Neonicotinoid resistant western flower thrips and tobacco thrips ○ Old world bollworm (Helicoverpa armigera) ○ Bermudagrass stem maggot (Atherigona reversura) ○ Crapemyrtle bark scale (Eriococcus lagerstroemiae) ○ Emerald ash borer (Agrilus planipennis) ○ Tawny Crazy Ant (Nylanderia fulva) and other invasive ants DISEASES: ○ QoI Cercospora Blight