Chapter 1 Regulation of Gastrointestinal Function, Appetite and Energy Intake 1.1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Victorian Historical Journal
VICTORIAN HISTORICAL JOURNAL VOLUME 89, NUMBER 2, DECEMBER 2018 ROYAL HISTORICAL SOCIETY OF VICTORIA VICTORIAN HISTORICAL JOURNAL ROYAL HISTORICAL SOCIETY OF VICTORIA The Royal Historical Society of Victoria is a community organisation comprising people from many fields committed to collecting, researching and sharing an understanding of the history of Victoria. The Victorian Historical Journal is a fully refereed journal dedicated to Australian, and especially Victorian, history produced twice yearly by the Publications Committee, Royal Historical Society of Victoria. PUBLICATIONS COMMITTEE Judith Smart and Richard Broome (Editors, Victorian Historical Journal) Jill Barnard Rozzi Bazzani Sharon Betridge (Co-editor, History News) Marilyn Bowler Richard Broome (Convenor) (Co-Editor, History News) Marie Clark Jonathan Craig (Review Editor) Don Garden (President, RHSV) John Rickard Judith Smart Lee Sulkowska Carole Woods BECOME A MEMBER Membership of the Royal Historical Society of Victoria is open. All those with an interest in history are welcome to join. Subscriptions can be purchased at: Royal Historical Society of Victoria 239 A’Beckett Street Melbourne, Victoria 3000, Australia Telephone: 03 9326 9288 Email: [email protected] www.historyvictoria.org.au Journals are also available for purchase online: www.historyvictoria.org.au/publications/victorian-historical-journal VICTORIAN HISTORICAL JOURNAL ISSUE 290 VOLUME 89, NUMBER 2 DECEMBER 2018 Royal Historical Society of Victoria Victorian Historical Journal Published by the Royal Historical Society of Victoria 239 A’Beckett Street Melbourne, Victoria 3000, Australia Telephone: 03 9326 9288 Fax: 03 9326 9477 Email: [email protected] www.historyvictoria.org.au Copyright © the authors and the Royal Historical Society of Victoria 2018 All material appearing in this publication is copyright and cannot be reproduced without the written permission of the publisher and the relevant author. -

Severe Storms on the East Coast of Australia 1770–2008
SEVERE STORMS ON THE EAST COAST OF AUSTRALIA 1770 – 2008 Jeff Callaghan Research Fellow, Griffith Centre for Coastal Management, Griffith University, Gold Coast, Qld Formerly Head Severe Storm Forecaster, Bureau of Meteorology, Brisbane Dr Peter Helman Senior Research Fellow, Griffith Centre for Coastal Management, Griffith University, Gold Coast, Qld Published by Griffith Centre for Coastal Management, Griffith University, Gold Coast, Queensland 10 November 2008 This publication is copyright. Apart from any fair dealing for the purpose of private study, research, criticism or review, as permitted under the Copyright Act, no part may be reproduced by any process without written permission from the publisher. ISBN: 978-1-921291-50-0 Foreword Severe storms can cause dramatic changes to the coast and devastation to our settlements. If we look back through history, to the first European observations by James Cook and Joseph Banks on Endeavour in 1770, we can improve our understanding of the nature of storms and indeed climate on the east coast. In times of climate change, it is essential that we understand natural climate variability that occurs in Australia. Looking back as far as we can is essential to understand how climate is likely to behave in the future. Studying coastal climate through this chronology is one element of the process. Analysis of the records has already given an indication that east coast climate fluctuates between phases of storminess and drought that can last for decades. Although records are fragmentary and not suitable for statistical analysis, patterns and climate theory can be derived. The dependence on shipping for transport and goods since European settlement ensures a good source of information on storms that gradually improves over time. -
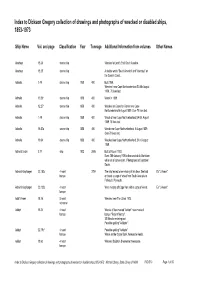
Index to Dickson Gregory Collection of Drawings and Photographs of Wrecked Or Disabled Ships, 1853-1973
Index to Dickson Gregory collection of drawings and photographs of wrecked or disabled ships, 1853-1973 Ship Name Vol. and page Classification Year TonnageAdditional Information from volumes Other Names Abertaye 18.36 steam ship Wrecked at Land's End, South America. Abertaye 18.25 steam ship A double wreck "South America" and "Abertaye" on the Cornish Coast. Admella 1.49 steam ship 1858 400 Built 1858. Wrecked near Cape Northumberland SA 6th August 1859, 70 lives lost. Admella 15.26* steam ship 1858 400 Wreck in 1859. Admella 12.27* steam ship 1858 400 Wrecked on Carpenter Rocks near Cape Northumberland 6 August 1859. Over 70 lives lost. Admella 1.49 steam ship 1858 400 Wreck of near Cape Northumberland SA 6th August 1859 70 lives lost. Admella 18.52a steam ship 1858 400 Wreck near Cape Northumberland, 6 August 1859. Over 70 lives lost. Admella 19.54 steam ship 1858 400 Wrecked near Cape Northumberland, SA, 6 August 1859. Admiral Cecile 3.77 ship 1902 2695 Built at Rouen 1902. Burnt 25th January 1925 in the canal de la Martiniere while out of commission. Photograped at Capetown Docks. Admiral Karpfanger 23.152c 4 mast 2754 The ship feared to be missing at this time. She had Ex "L'Avenir". barque on board a cargo of wheat from South Australia to Falmouth, Plymouth. Admiral Karpfanger 23.132c 4 mast Went missing off Cape Horn with a cargo of wheat. Ex "L'Avenir". barque Adolf Vinnen 18.14 5 mast Wrecked near The Lizard 1923. schooner Adolph 18.34 4 mast Wrecks of four masted "Adolph" near masts of barque barque "Regent Murray". -

Tony Brady : Wendouree, Clare Valley
Tony Brady : Wendouree, Clare Valley Wendouree is considered by many wine lovers to be among the most beautiful and distinctive wines this country has to offer. Wendouree Shiraz. Years ago we gathered for a weekend in Mildura, with a group of our friends from the eastern states, mainly to have a night at Stefano de Pieri’s restaurant, Stefano’s. When I asked about BYO, the manager said, “we normally only allow one bottle per table sir”. I took along a magnum of 1990 Wendouree Shiraz. On the night he said, “If you’d have told me you were bringing Wendouree, I would have allowed some more, so long as we got a glass to share.” Tony Brady and his wife Lita have been the custodians of Wendouree since 1974. Here’s Tony’s story Wendouree has been producing wine for a very long time. Who originally started it and when ? Alfred Percy Birks ‘AP Birks’ started in 1892. Roly Birks as a young boy with his family. Roly is on the grass at the front. His father was a medico in Angaston. AP was one of four boys. He and one of his brothers planted a 0.2-ha block of cabernet sauvignon. One of his other brothers was Charles Birks, of the Adelaide Department store Birks. There was a story that it started out as a hobby, but gee they worked hard. It’s remarkable what varieties they planted and where, just how astute time has shown them to be. We assume they had not much knowledge of growing grapes, but we forget a lot of the pastoralists in those days were Oxford or Cambridge educated. -

Winelist 1St March.Numbers
Cocktails Berowra Waters Inn Signature Australian Cocktails $23 Beers Happy Goblin, Pale Ale Mt Kuring-Gai, NSW $12 Young Henrys “Newtowner”, Pale Ale Newtown, NSW $11 La Sirène, Saison Melbourne, VIC $16 Cider Napoleone, Pear Cider Yarra Valley, VIC $11 Soft Drinks | Mocktail P.S Soda, Wattle Cola Sydney. NSW $8 P.S Soda, Blackstrap Ginger Sydney, NSW $8 Strange Love, Blood Orange & Chili Byron Bay, NSW $7.5 Seasonal Mocktail Sydney, NSW $12 Gin/Tequila 30ml ( + $3 with Strange Love | P.S Soda) Applewood Distillery Gin Adelaide Hills, SA $13 Hippocampus Metropolitan Distillery, Gin Perth, WA $14 Espólon Blanco Tequila Arandas, Mexico $12 Vermouth 60ml Adelaide Hills Distillery, Dry Vermouth Adelaide Hills, SA $9 Vodka 30ml ( + $3 with Strange Love mixer ) Twenty Third St. Distillery, Riverland Rose Vodka Renmark, SA $13 Archie Rose Distillery, Original Rosebery, NSW $12 Wines by the glass Sparkling 100ml 2014 Caledonia Australis “Umbra” Blanc de Blanc South Gippsland, VIC $20 NV Dominique Portet, Brut Rosé Yarra Valley, VIC $15 White 150ml 2017 Nick Spencer, Grüner Veltliner Tumbarumba, NSW $16 2016 Geyer, Muscat Alexandrie Barossa Valley, SA $14 2015 Moon, Marsanne Nagambie, VIC $18 2015 Dalrymple “Cave Block”, Chardonnay Pipers River, Tasmania $17 Coravin 2014 Christian Moreau Grand Cru Le Clos Chablis, France $41 Rosé 150ml 2017 Nocturne, Nebbiolo Margaret River, WA $17 Red 150ml 2015 Lino Ramble “Tom Bowler”, Nero d’Avola McLaren Vale, SA $18 2016 Yelland & Papps “Second Take”, Grenache Barossa, SA $18 2016 Inkwell “Infidels”, Primitivo -

Gortatlea, Ballymacelligott, Co.Kerry
BLENNERHASSETT family of ASH HILL, GORTATLEA, BLACKBRIAR, GLENAGEENTY, RATHANNY, BALLYMACELLIGOTT & BEAUFORT in Co.KERRY Blennerhassett Family Tree (BH09_Gortatlea_G.xlsx) revised December 2013, copyright © Bill Jehan 1968-2013 Thanks to all who have contributed to these pages - please email additions & corrections to: [email protected] DISINHERITANCE According to family tradition, had the entail on the Ballyseedy estate been conventionally followed, THIS PAGE CONTINUES FROM page B d.196206 of: Brisban; and bur. Nudgee THISCem., PAGEBrisbane CONTINUES FROM page K 50 on: the heirs of Thomas Blennerhassett of Ash Hill, Ballymacelligott (b.18.12.1736) would have inherited; Blennerhassett of BALLYSEEDY, Co.Kerry Blennerhassett of CASTLE CONWAY, KILLORGLIN, Co.Kerry This did not happen because the 1774 will of "The Great Colonel John" Blennerhassett (p.B 01), while recording the Ballyseedy entail in great detail, completely omitted to include Thomas. From this Gortatlea family descends: Blennerhassett of LISSATAGGLE, CURRANS, also GLENDINE, CAMP, KILGOBBAN, Co.Kerry The disinheritance is perhaps because of Thomas' inappropriate marriage, but that is uncertain. G 01 ASH HILL, BALLYMACELLIGOTT SERJEANT THOMAS "TOM" BLENNERHASSETT Valerie Bary in [HK] tells us: "...Ash Hill House was built in 1700 Thomas Arthur Blennerhassett was Permanent Serjeant & Paymaster of the volunteer "Kerry County Yeomanry" by the Blennerhassetts, who lived there for a long time. Elmgrove & Blennerville Unit, 1823-34; Arthur Blennerhassett of Ballyseedy -

Presidents Report 2020.Pdf
Wendouree Ballarat Rowing Club Annual Report 2019 -2020 I would like to begin by thanking all those present for continuing to show their support. I will begin by reminding everyone of the Wendouree Ballarat Rowing Club Vision. ‘We want to create an environment that fosters rowing in the local community and provides rowers with a pathway and opportunity to compete and be involved on the National & World Stage.’ As evidenced by the AGM being presented online, I think it is safe to say this year is like no other year on record. We have been dominated by one topic this year, COVID-19. Some say that it is not dissimilar to the Spanish flu in 1919 however when I look back through the records the club recorded one of its best membership levels ever in 1919. Fingers crossed we have a similar result however the lockdown remains, and the club is closed until mid-September. The interruption to our sport of rowing began on Friday the 13th of March. After a day of competition at the Head of Schoolgirls an announcement was read out to the dismay of the rowing community. The balance of the regatta had been suspended. With out any knowledge of what was to come some felt this was an overreaction. March 14 the Australian Government advises against gatherings of 500 people or more. March 16 Victoria declares a state of emergency and the State goes into lockdown. The affect of this lockdown and world outbreak puts sport on hold. All national and international events were suspended not long after the first lockdown. -

Banking Act Unclaimed Money As at 31 December 2007
Commonwealth of Australia Gazette No. ASIC 40A/08, Wednesday, 21 May 2008 Published by ASIC ASIC Gazette Contents Banking Act Unclaimed Money as at 31 December 2007 RIGHTS OF REVIEW Persons affected by certain decisions made by ASIC under the Corporations Act 2001 and the other legislation administered by ASIC may have rights of review. ASIC has published Regulatory Guide 57 Notification of rights of review (RG57) and Information Sheet ASIC decisions – your rights (INFO 9) to assist you to determine whether you have a right of review. You can obtain a copy of these documents from the ASIC Digest, the ASIC website at www.asic.gov.au or from the Administrative Law Co-ordinator in the ASIC office with which you have been dealing. ISSN 1445-6060 (Online version) Available from www.asic.gov.au ISSN 1445-6079 (CD-ROM version) Email [email protected] © Commonwealth of Australia, 2008 This work is copyright. Apart from any use permitted under the Copyright Act 1968, all rights are reserved. Requests for authorisation to reproduce, publish or communicate this work should be made to: Gazette Publisher, Australian Securities and Investment Commission, GPO Box 9827, Melbourne Vic 3001 ASIC GAZETTE Commonwealth of Australia Gazette ASIC 40A/08, Wednesday, 21 May 2008 Banking Act Unclaimed Money Page 2 of 463 Specific disclaimer for Special Gazette relating to Banking Unclaimed Monies The information in this Gazette is provided by Authorised Deposit-taking Institutions to ASIC pursuant to the Banking Act (Commonwealth) 1959. The information is published by ASIC as supplied by the relevant Authorised Deposit-taking Institution and ASIC does not add to the information. -

1990 2Nd Quarter
The Philatelic Communicator Journal of Writers Unit 30 of the American Philatelic Society ISSN 0147-3646 Volume 23, No. 2, Whole Number 88, Second Quarter, 1990. The President’s Message Strategies for Topical Writing By Robert de Violini By Ken Lawrence As soon as I got the First Quarter issue for 1990 it was time Topical stamp collecting—also known as thematic philate- to prepare this column for the Second Quarter. Let’s hope that ly*—is the wave ofthe present and the future. It has not Joe has had some better hick with Epson America in getting his displaced traditional collecting or postal history, but its popularity laser printer fixed than he has had so far. And also let us hope is greater than our literature reflects. that we can get back on track with a more regular schedule of To demonstrate this, I present as my first witness Richard W. publication of The Philatelic Communicator. It doesn’t help Helbock, writing in his flagship journal. La Posta: A Journal of attendance to read of a “ coming event’’ several weeks after it American Postal History. Though he’s a cover lover of the was held. traditional mold, Helbock is crusading to save our hobby from There isn’t anything wrong with Epson laser printers—the the threats that surround us on every side—from baseball cards new low-cost EPL-6000 model has received excellent re- tocomicbooks. ))Page35 views—it’s just that Joe Frye has one of their older models that has begun to show its age, and it has become difficult to get the * At the lofty level of national and international exhibiting in competition, people at Epson’s repair center to do their job correctly.* thematic has a distinct technical meaning, referring to the preferred methodof The current plans are for two issues of the PC between now organizing a topical collection for display. -
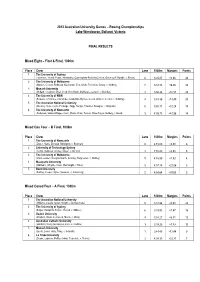
2013-Rowing-Results.Pdf
2013 Australian University Games – Rowing Championships Lake Wendouree, Ballarat, Victoria FINAL RESULTS Mixed Eight – First & Final, 1000m Place Crew Lane 1000m Margins Points 1 The University of Sydney (Lawrence, Yerrell, Evans, Winstanley, Cunningham-Reid, McClintock, Belonogoff, Rundle, c: Raven) 6 3:25.87 +0.00 48 2 The University of Melbourne (Watson, Cooper, Redhead, Mackenzie, Ellis, Smith, Treverton, Cleary, c: Webley) 7 3:32.33 +6.46 32 3 Monash University (Mollard, Houghton, Dyer, Cook, Nott, Bush, Matthews, Jackson, c: Brander) 2 3:36.44 +10.57 24 4 The University of NSW (Flowers, Johnstone, Johnstone, Jerapetritis, Byrnes, Couch, Watson, Cooms, c: Schilling) 4 3:45.36 +19.49 20 5 The Australian National University (Wookey, Rose, Lyons, Furmage, Tagg, Raniga, Thwaites, Basaglia, c: Kingham) 5 3:58.11 +32.24 18 6 The University of Newcastle (Pedersen, Staines-Magee, Conn, Wade, Shaw, Tentori, Shaw-Taylor, Boldery, c: Bond) 3 4:08.73 +42.86 14 Mixed Cox Four – B Final, 1000m Place Crew Lane 1000m Margins Points 1 The University of Newcastle (Dwyer, Saltis, Dunstan, Rimington, c: Bowman) 6 4:15.09 +0.00 6 2 University of Technology Sydney (Temm, Delipetar, Aroney, Mayer, c: De Vos) 4 4:15.89 +0.80 5 3 The University of Melbourne (Van Leeuwen, Douglas-Scarfe, Goodey, Hargreaves, c: Webley) 5 4:16.99 +1.90 4 4 Macquarie University (Whittaker, Whyntie, Clark, Wainwright, c: Niles) 3 4:37.15 +22.06 3 5 Bond University (Purling, Cooper, Wylie, Stawaruk, c: Armstrong) 2 4:54.64 +39.55 2 Mixed Coxed Four – A Final, 1000m Place Crew -

BALLARAT HERITAGE PRECINCTS STATEMENTS of SIGNIFICANCE 2006 (Revised August 2014)
BALLARAT HERITAGE PRECINCTS STATEMENTS OF SIGNIFICANCE 2006 (revised August 2014) This document relates to the following Heritage Precincts in the Ballarat Planning Scheme Heritage Overlay: Lake Wendouree Map Ref HO 163 West Ballarat Map Ref HO 164 Victoria Park Map Ref HO 165 Central Ballarat Map Ref HO 166 Sturt Street Map Ref HO 167 South Ballarat Map Ref HO 168 Waller Estate Map Ref HO 169 Soldiers Hill Map Ref HO 170 Lydiard Street Map Ref HO 171 Creeks & Rivers Channels Map Ref HO 172 Mount Pleasant/Golden Point Map Ref HO 173 Black Hill Map Ref HO 174 Humffray Street Map Ref HO 175 Bridge Mall/Bakery Hill Map Ref HO 176 Victoria Street Map Ref HO 177 Ballarat East Civic Map Ref HO 178 Eureka Street Map Ref HO 179 Learmonth Map Ref HO 180 Buninyong Map Ref HO 181 This document is to be used in conjunctIon with the draft Guidelines for the Assessment of Heritage Planning Applications 2000. Acknowledgements Ballarat City Council wishes to acknowledge the contribution of the authors of the Ballarat Heritage Study Stage 2 , 2003 report. ƒ Hansen Partnership Pty Ltd. ƒ Wendy Jacobs, Architect and Heritage Consultant. ƒ Naga Services. ƒ Dr Jan Penney. ƒ Ms Vicki Johnson. Ms Amanda Jean as the author of the revised Statements of Significance contained in this report. The Statement of Significance for the Victoria Street Precinct (HO177) was refreshed by Dr David Rowe following the recommendations in the Panel Report for Amendment C164 to the Ballarat Planning Scheme. Table of Contents Introduction ....................................................................................................................... 1 LAKE WENDOUREE HERITAGE PRECINCT ................................................................ -

Orora Limited – Head Office Orora Innovation & Design
ORORA LIMITED – HEAD OFFICE ORORA INNOVATION & DESIGN CENTRE 109 Burwood Road 109 Burwood Road Hawthorn VIC 3122 Hawthorn Vic 3122 Australia Tele: +61 3 9811 7167 Tel: +61 3 9811 7111 Fax:+61 3 9811 7171 ORORA BEVERAGE Sales Enquiries AUSTRALIA: Beverage Cans +61 3 9811 7459, 9811 7439 or 9811 7289 Closures (Plastic) +61 3 9811 7459, 9811 7439 or 9811 7289 Stelvin Closures (Wine) +61 8 8433 2510 or 8433 2514 or email: [email protected] Glass +61 8 8521 4600 NEW ZEALAND: Beverage Can +64 9 262 6977 Stelvin Closures (Wine) email: [email protected] Glass +61 8 8521 4600 or email: [email protected] Manufacturing Sites Orora Beverage Cans - Canning Vale Orora Beverage Cans - Dandenong 153 Bannister Road Gate 1, 100 South Gippsland Highway Canning Vale WA 6155 Dandenong VIC 3175 Australia Australia Tel: +61 8 9256 9300 Tel: +61 3 9767 5757 Orora Beverage Cans - Revesby Orora Beverage Can Ends - Wendouree 146 Carrington Street 83 Warringa Dve Revesby NSW 2212 Wendouree VIC 3355 Australia Australia Tel: +61 2 9772 3566 Tel: +61 3 5330 7111 Orora Beverage Cans - Rocklea Orora Glass - Australasia 75 Ashover Road 48 Argent Road Rocklea QLD 4016 Kingsford SA 5118 Australia Australia Tel: +61 7 3722 4888 Tel: +61 8 8521 4600 Orora Closures - Dudley Park Orora Beverage Cans - Wiri 65-75 Pym Street Dudley Park 17-19 Ash Road Adelaide SA 5008 Wiri Auckland Australia New Zealand Tel: +61 8 8433 2500 Tel: +64 9 262 3192 ORORA FIBRE PACKAGING (OFP) Sales Enquiries AUSTRALIA: Corrugated boxes (fruit boxes, cartons,