Lakes Rerewhakaaitu and Ōkaro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
There's a Lot More To
THERE’S A LOT MORE TO FISH&GAMENZ MAGAZINE AND ONLINE THAN JUST THE SPECIAL ISSUES issueninetyfive FISH & GAME NEW ZEALAND New Zealand $9.90 incl GST issueninetysix FISH & GAME NEW ZEALAND THE AUTHORITY ON FRESHWATER FISHING AND GAME BIRD HUNTING IN NEW ZEALAND New Zealand $9.90 incl GST issue ninetyseven New Zealand $9.90 incl GST BROADEN YOUR ANGLING HORIZONS The Best Month For Fishing ISSUE NINETY FIVE As Good As It Gets Capturing Aerial Antics Moods Of The Tutaekuri JANUARY 2017 22/12/16 2:57 pm ISSUE NINETY SIX So You ThinkYou Can Cast F&G cover Iss95.indd 1 The Fortuity Of Fly Fishing APRIL 2017 Moods Of Bridges I SSUE N 4/04/17 12:34 pm We’re Fishing I Where NETY Red Rock Trout SEVEN F&G cover Iss96.indd 1 Post-Season Shakedown JULY Cracking The Canal Code 2017 Brothers In Arms CATCH THE OTHER FOUR & DON’T MISS OUT! Check out the latest subscription deal online at www.fishandgamenz.co.nz - today! BDMAREVOLUTION_ J000140 J000140_advert.indd 1 17/07/17 3:05 pm Fish & Game 1 2 3 5 4 6 Check www.fishandgame.org.nz for details of regional boundaries Code of Conduct ....................................................................... 4 National Sports Fishing Regulations ..................................... 5 First Schedule ............................................................................ 7 1. Northland ............................................................................ 11 2. Auckland/Waikato ............................................................ 14 3. Eastern ................................................................................. -

Part 2 Geological History.Indd
2. Geological History Activities Curriculum Curriculum Environmental Page Activity title level link education aspect 2a Formation of the L 3–5 Science 37 Rotorua lakes 2b Silent Card Shuffl e L 3–5 Science 39 2c Mt Ngongotaha L 3–5 Science About, In 42 2d Looking at Sediment Any level Science About, In 53 2e How Wet is your Any level Science 55 Sediment? 2f What is in your L 5 Science 58 Sediment? Relevant resources: • Rotorua’s Volcanic Past – video – hire from Rotorua Public Library • Rotorua Museum Education Resources » Te Arawa – Mai Maketu ki Tongariro » Legends, Landforms and Learning » Tarawera photo kit » Tarawera Resource kit • Pollution Busters newsletter # 5 Volcanoes • Learning Media - Building Science Concepts • Book 12 – Volcanoes: Hot Rock in a Cool World Levels: 3–4 • Book 52 – The Land Changes: Keeping Earth’s Systems in Balance: Levels: 3–4 • Learning Media – Readers » The Changing Land, Jane Buxton, ISBN 0478214162, explores how the elements can affect Earth and change its shape The Rotorua Lakes Education Resource 35 Activity 2a Formation of the Rotorua Lakes Curriculum links Science Any level 2 Resources required Science • PowerPoint presentation by Will Esler, University of Waikato (on CD) • The Life and Times of Lake Rotorua and Lake Rotoiti (see background notes) • Graphic of Rotorua lake edge 9000 years ago Method 1 View the PowerPoint (ppt) before using it with the class, or set a small group this responsibility. Identify good stopping places for discussion or questions. Have some questions already identifi ed e.g. What was the signifi cant event during this time? Why was it signifi cant? Who/what was responsible for this event occurring? If a small group has previewed the ppt then they can act as a lead questioner/facilitator for one of the groups below. -

Game Bird Hunting Areas Rotorua Lakes
Game bird hunting areas Rotorua lakes Eastern Region Lake Rotoiti Lake Rotorua Open to hunting (public land) Closed to hunting Other areas of private land might be available to be hunted by gaining permission of landowner. Lake Rotorua Open to game bird hunting with some closed areas as indicated on map. These areas are Sulphur Bay, Hamurana Stream and the lake within a radius of 800 metres of the Hamurana stream mouth and Mokoia Island plus a radius of 100 metres off the Island. When hunting on Lake Rotorua be aware of built-up areas and those used by the public and your responsibility pursuant to the Arms Act 1983. (see over) Lake Rotoiti Open to game bird hunting. Shore based hunting is only permitted on the sections of Lake Rotoiti Scenic Lake Rotoehu Reserve identified on the map, and a DOC game bird hunting permit is required. Maimais are not permitted on Lake Rotoiti. Lake Rotoehu Open to game bird hunting. Shore based hunting is only permitted on the sections of Lake Rotoehu Scenic Reserve identified on the map, and a DOC game bird hunting permit is required. Temporary maimais may be constructed but no earlier than two weeks before the beginning of the game bird hunting season. All temporary structures must be removed no later than two weeks after the close of the game bird hunting season. Lake Rotoma Open to game bird hunting. Shore based hunting is only permitted on the sections of Lake Rotoma Scenic Reserve identified on the map, and a DOC game bird hunting permit is required. -

Adapting to Climate Change in Rerewhakaaitu
Adapting to climate change in Rerewhakaaitu A history of change at Rerewhakaaitu Background 400,000 years (at least) of volcanic activity, geological changes, and Lake Rerewhakaaitu lies within the climate changes Okataina Volcanic Centre where volcanic ca 17,600 years ago Volcanic activity and climate change eruptions have featured for at least the last 400,000 years (see Nairn, http: ca 800 years ago Maori settlement in New Zealand //www.gns.cri.nz/what/earthact/volcanoes/ 700 years ago Lake Rerewhakaaitu formed from the nzvolcanoes/okatbookprint.htm). Volcanic Kaharoa eruption of Mt Tarawera activity has been and will continue to be 140 years ago First recorded European settlement a huge factor in shaping the landscape, 120 years ago Most recent Tarawera eruption land use and water features in this area. Concurrently, past climate changes have 60 years ago Post-war farm ballots and development of Rerewhakaaitu also had a strong influence. Evidence of the end of the last glaciation and 40 years ago Local flooding and erosion problems onset of the current interglacial period The last 60 years Development (1950s and 60s), consolida- is found in the Rerewhakaaitu Tephra, tion (1970s), and progressive intensification arising from a Tarawara eruption about (1980s to present) of farming 18,000 – 17,400 years ago (Newham et The future ? al, 2003). PAGE • ADAPTING TO CLIMATE CHANGE IN EASTERN NZ Meso Adaptation Meso Adaptation ADAPTING TO CLIMATE CHANGE IN EASTERN NZ • PAGE 117 The most recent volcanic eruption, ‘Development experienced in the 1960s from Tarawera in 1886, has strongly of farming at and 1970s which is docu- influenced the present environment mented in correspondence at Rerewhakaaitu. -

Reducing Nutrient Losses to Lake Rerewhaakitu
REDUCING NUTRIENT LOSSES TO LAKE REREWHAAKITU Bob Longhurst 1, Ian Power 1, Martin Hawke 2, Bob Parker 3 1 AgResearch Ruakura, Hamilton 2 Bay of Plenty Farm & Pastoral Research, Rotorua 3 Fruition Horticulture, Tauranga Abstract Lake Rerewhakaaitu is a mesotrophic shallow lake, unique amongst the Rotorua Lakes, for having a catchment comprising mostly dairy farms. Water quality, as measured by the trophic level index (TLI), has in recent years gone above the Bay of Plenty Regional Council‟s TLI target of 3.6. Farmers within the catchment are concerned about: 1) the future condition of the lake and 2) possible imposition of constraints on their farming operations. During the past eight years Sustainable Farming Fund projects have been set up to address water quality issues by identifying ways that pastoral management in the catchment could be changed to minimise the environmental impact on the lake, and allow sustainable dairy farming to continue. In Phase 1 a farmer survey was conducted and OVERSEER® nutrient budgets were compiled for each farm in the lake‟s catchment. Nitrogen management was the main priority. Phase 2 focused on phosphorus (P) management, identifying the main pollutant form of P so that appropriate on-farm P-mitigations could be demonstrated. In Phase 3 OVERSEER® nutrient budgets were again compiled for each farm and a „Farm Enviro Walk‟ undertaken to identify critical source areas for nutrient loss. On-farm mitigations have been identified for farmers to implement. The collective response of farmer mitigations has seen total modelled N and P nutrient loadings from the catchment between 2002/03 and 2009/10 reduced by 18% and 28% respectively. -

Assessment of the Rotorua Te Arawa Lakes Using Lakespi - 2015
Taihoro Nukurangi Assessment of the Rotorua Te Arawa lakes using LakeSPI - 2015 Prepared for Bay of Plenty Regional Council September2015 NIWA - enhancing the benefits of New Zealands natural resources wwwniwaco.nz Prepared by: Tracey Burton John Clayton For any information regarding this report please contact: Tracey Burton Freshwater Ecologist Aquatic Plants +64-7-856 7026 tracey.burton@ niwa.co.nz National Institute of Water & Atmospheric Research Ltd PU Box 11115 Hamilton 3251 Phone +64 7 856 7026 NIWA CLIENT REPORT No: HAM2015-091 Report date: September 2015 NIWA Project: B0P15203 Quality Statementiit1i1 Reviewed by: Mary de Winton Formatting checked by: Alison Bartley Approved for release by: David Roper © All rights reserved. This publication may not be reproduced or copied in any form without the permission of the copyright owner(s). Such permission is only to be given in accordance with the terms of the client's contract with NIWA. This copyright extends to all forms of copying and any storage of material in any kind of information retrieval system. Whilst NIWA has used all reasonable endeavours to ensure that the information contained in this document is accurate, NIWA does not give any express or implied warranty as to the completeness of the information contained herein, or that it will be suitable for any purpose(s) other than those specifically contemplated during the Project or agreed by NIWA and the Client. Contents Executivesummary ............................................................................................................ -

Bay of Plenty Inter-Middle Schools Cross Country Championships 2014 - Results
BAY OF PLENTY INTER-MIDDLE SCHOOLS CROSS COUNTRY CHAMPIONSHIPS 2014 - RESULTS Placing Time Year 4 Girls School Region 1 6.17.48 Moana Manukau Kawerau South EBOP 2 6.19.83 Kaia Skipps Awakeri EBOP 3 6.27.25 Nivaloh Elliott Kawerau South EBOP 4 3.31.15 Anna Issac Rangitaiki Ind EBOP 5 6.32.14 Chevana Larsen James street EBOP 6 6.37.50 Ruby Valentine Greenpark WBOP 7 6.37.92 Cerys Adkins Golden Sands WBOP 8 6.39.92 Marissa Light Westbrook CBOP 9 6.41.20 Angela Rowson Otonga CBOP 10 6.42.64 Maya Stone Omokoroa No. 1 WBOP 11 Lulu Johnson Omokoroa No. 1 WBOP 12 Jenna Hodgson Welcome Bay WBOP 13 Lystelle Lepper Allandale EBOP 15 Kiara Dudson Westbrook CBOP 16 Evie Gibney Fairhaven WBOP 17 Tayla Brady St Josephs, Whk EBOP 18 Eima Seru Otonga CBOP 19 Sarah Edwards St Thomas More WBOP 20 Sophie Kirk Ohope EBOP 21 Lily Aitken-Keogh Tauranga Primary WBOP 22 Eden Beasley St Josephs, Whk EBOP 23 Sofia Vosse Kaharoa CBOP 24 Lucy Lloyd Matua WBOP 25 Breeze Franklin Ashbrook EBOP 26 Amelia Foley Selwyn CBOP 27 Ivy Mitchell Omanu WBOP 28 Caitlyn Esterhuizen Ohope EBOP 29 Hannah Shilton Lynmore CBOP 30 Te Aroha Healey-Forde Malfroy CBOP 31 Milla Pipps Rotokawa CBOP 32 Ella Mcsweeney Omanu WBOP 33 Mikaela Blackett Westbrook CBOP 34 Bonnie- Marie Wetting Awakeri EBOP 35 Jessica Cleghorn Allandale EBOP 36 Tyla Pope Westbrook CBOP 37 Jasmine Donaldson Lynmore CBOP BAY OF PLENTY INTER-MIDDLE SCHOOLS CROSS COUNTRY CHAMPIONSHIPS 2014 - RESULTS 38 Hine Lankshear Omokoroa No. -
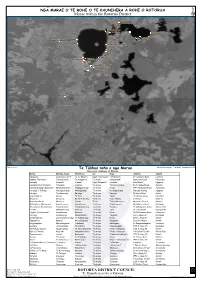
Marae Within the Rotorua District FF 2E2‚Yri2y2‚Y„Y‚…E Xqe2we‚Ei2y2„I2
Marae Meeting House Whare Kai Iwi Hapu Address Suburb Apumoana Apumoana 1 & 2 * Te Ao Wheoro Ngati Tumatawera Tumatawera 27 Tarawera Road Lynmore Awahou (Tarimano) Tawakeheimoa Te Aongahoro Te Arawa Rangiwewehi Hamurana Road Hamurana Hinemihi Hinemihi Hinewai Ngati Tarawhai* Hinemihi Hona Road Ngapuna Hinemoa Point (Owhata) Tutanekai Hinemoa Te Arawa Te Rorooterangi Iri-Irikapua Parad Owhata Hinetapaturangi (Waiatuhi) Kahumatamomoe Hinetapaturangi Te Arawa Pikiao 1444 Hamurana Road Hamurana Hurunga O Te Rangi Hurungaterangi Whaingarangi Te Arawa Hurungaterangi Hurunga Avenue Ngapuna Karenga Tumahaurangi Karenga Te Arawa Karenga 38 Koutu Road Koutu Kearoa Marae Kearoa Te Uira Te Arawa Tuara 25 Apirana Road Horohoro Mataarae Mataarae Ko Te Aroha Rau Te Arawa Ngati Whaoa S Hway 5 Reporoa Mataatua Marae Mataatua Hinetai Tuhoe Tuhoe/Mataatua Mataatua Street Rotorua Mataikotare (Waiohewa) Rangiteaorere Uenukurauiri Te Arawa Rangiteaorere Mataikotare Road Rotokawa Otaramarae (Pounamunui) Houmaitawhiti Hinekukuterangi Te Arawa Hinekura 97 Whangamoa Drive Okere Falls Parawai Whatumairangi Parehina Te Arawa Tura 19 Taui Street Ngongotaha Taupari (Paruaharanui) Paruaharanui Waiwaha Te Arawa Parua 1435 Hamurana Road Hamurana Pikirangi Ohomairangi Makuratawhiti Te Arawa Turipuku 932 S Hway 30 Rotokawa Punawhakareia Uenukumairarotongo Te Aokapurangi Te Arawa Pikiao 1270 S Hway 30 Rotoiti Tupuaekura Rakeiao Maruahangaroa Te Arawa Rongomai 111 Curtis Road Rotoiti Rongomaipapa Maruahangaroa Hineteata Te Arawa Kahungunu 46 Rongomaipapa Ro Horohoro Ruamata -

Pūrongo-Ā-Tau Annual Report 2020 2 ROTOITI 15 | Annual Report 2020 Tirotiro Contents
Pūrongo-ā-tau Annual Report 2020 2 ROTOITI 15 | Annual Report 2020 Tirotiro Contents Ngā hua o te tau | Highlights 4 Kaupapataka | Agenda 5 Ngā mīneti | Minutes of the 2019 AGM 6 Ngā Mātārae | Trustees 12 Ngā Tauwharenga | Management team 15 Ngā Ia o ngā Awa | Administration team 16 Te rautaki matua | Strategic directions 17 Pūrongo o Te Kūrae | Chairman’s report 18 Pūrongo o Te Kūrae Pakihi | 20 Chairman’s limited partnership report Pūrongo o Te Tauwharenga | 22 General Manager’s report Te pūtea tautoko | Distributions report 23 Whakarāpopototanga | 24 Summary of distributions Pūrongo ā pūtea | Financial report 25 Tauākī Pūtea | Audited Financial Statements 29 Ngā mahere | Maps 56 Tō matau tohu | Our tohu 59 ROTOITI 15 | Annual Report 2020 ROTOITI 15 | Annual Report 2020 3 Ngā hua o te tau Highlights 15,826 Rotoiti 15 Share holders $412,743 $ Grants paid for the year $54.9m Total Asset Base 4 Kaimahi 4 ROTOITI 15 | Annual Report 2020 Kaupapataka Agenda 2020 HUI- Ā- TAU ANNUAL GENERAL MEETING Tapuaekura Marae Sunday 22nd November 2020 at 10.00am (Registrations commence at 9.00am) 1. Minutes 2. Election of ONE trustee 3. Chairman’s Trust Report 4. Chairman’s Limited Partnership Report 5. General Manager’s Report 6. Financial Report 7. Appointment of Auditor 8. Distributions Report and Review of Distribution policy 9. Environmental Policy 10. General Business ROTOITI 15 | Annual Report 2020 ROTOITI 15 | Annual Report 2020 5 Ngā mīneti Minutes of the 2019 AGM ANNUAL GENERAL MEETING Karakia that the issue she raised was in regards to correct process and methodology being OF OWNERS Tumihitai Raerino outdated, rather than a trust issue. -

Mfeprojectedwaterqualityforswim
Water quality for swimming 2030 Excellent Good Fair Intermittent Poor * Specified rivers are those classified as fourth order and above. Specified lakes are those with a perimeter of 1.5km or more MfE projected water quality for swimming - 2030 Projection and Grid Information HORIZONTAL DATUM: New Zealand Geodetic Datum 2000 Specified* Rivers and lakes in Bay of Plenty Region For practical purposes, NZGD2000 equates to WGS84 VERTICAL DATUM: Moturiki PROJECTION: New Zealand Transverse Mercator 2000 Scale1:775,000 GSP-571615 © Bay of Plenty Regional Council, 2018 10 0 10 20 30 © Sourced from Land Information New Zealand data. Sheet 1 of 1 CROWN COPYRIGHT RESERVED Kilometres Printed 21/11/2018 List of Specified* Rivers and Lake names identified within the MFE maps for the Bay Of Plenty region by Water Management Area (WMA) *Specified rivers are those classified as fourth order or above. Specified lakes are those with a perimeter of 1.5 kilometres or more. RIVERS East Coast Water Management Area Kaituna-Pongakawa-Waitahanui WMA Rangitaiki WMA continued Rotorua Lakes WMA continued Length (Km) Length (Km) Length (Km) Name of River Length (Km) Name of River Name of River Name of River Haparapara River 27.8 Kaikokopu Canal 5.3 Otamatea River 27.1 Tupapakurua Stream 4.0 Hawai River 11.5 Kaituna River 49.2 Otangimoana Stream 12.6 Tureporepo Stream 1.1 Kahoka Stream 7.7 Mangorewa River 31.4 Pekepeke Stream 11.5 Umurua Stream 5.5 Kereu River 24.7 Oeuteheuheu Stream 4.0 Pokairoa Stream 6.3 Utuhina Stream 10.9 Mangahinatore Stream 2.1 Onaia Stream -

Groundwater in the Okataina Caldera: Model of Future Nitrogen Loads to Lake Tarawera
Groundwater in the Okataina caldera: Model of future nitrogen loads to Lake Tarawera CBER Report 94 Prepared for Environment Bay of Plenty and Lake Tarawera Ratepayers’ Association By Nicolas Gillon1, Paul White2, David Hamilton1 and Warwick Silvester1 1. Centre for Biodiversity and Ecology Research, School of Science and Engineering, The University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand 2. Institute of Geological and Nuclear Sciences, Private Bag 2000, Taupo, 3352, New Zealand September 2009 1 Table of contents List of figures……………………………………………………………………………. 4 List of tables………………………………………………………………………………6 1. Introduction ................................................................................................... 6 2. Geomorphology and Land Use .................................................................... 7 2.1 Okataina Volcanic Centre ................................................................................................ 7 2.2 Lake Tarawera surface catchment ................................................................................... 9 3. Geology ......................................................................................................... 12 3.1 Geological history and lithological description of the Okataina Caldera complex ....... 12 3.1.1 Whakamaru Ignimbrite ......................................................................................................... 12 3.1.2 Matahina Ignimbrite ............................................................................................................ -

NIWA Client Report No: HAM2014-067 Report Date: June 2014 NIWA Project: BOP14203
Assessment of the Rotorua Te Arawa lakes using LakeSPI - 2014 Prepared for Bay of Plenty Regional Council June 2014 Authors/Contributors: Tracey Burton John Clayton For any information regarding this report please contact: Tracey Burton Freshwater Ecologist +64-7-8569 1852 [email protected] National Institute of Water & Atmospheric Research Ltd Gate 10, Silverdale Road Hillcrest, Hamilton 3216 PO Box 11115, Hillcrest Hamilton 3251 New Zealand Phone +64-7-856 7026 Fax +64-7-856 0151 NIWA Client Report No: HAM2014-067 Report date: June 2014 NIWA Project: BOP14203 Contents Executive summary .............................................................................................................. 7 1 Introduction ................................................................................................................. 7 1.1 Background .......................................................................................................... 9 1.2 Study lakes .......................................................................................................... 9 1.3 History of the Rotorua Lakes .............................................................................. 11 2 Study methods .......................................................................................................... 14 2.1 LakeSPI ............................................................................................................. 14 2.2 Field surveys .....................................................................................................