Repetitive TMS of the Motor Cortex Improves Ipsilateral Sequential Simple Finger Movements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atrial Septal Defect (ASD) – Disorder of the Heart That Is Present at Birth Involving a Hole in the Wall (Septum) Separating the Two Upper Chambers (Atria)
Glossary of medical terms (grouped by affected system or organ) Atrial septal defect (ASD) – disorder of the heart that is present at birth involving a hole in the wall (septum) separating the two upper chambers (atria) Ventricular septal defect (VSD) – disorder of the heart that is present at birth involving a hole in the wall (septum) separating the two lower chambers (ventricles) Patent ductus arteriosus (PDA) – failure of the ductus arteriosus, an arterial shunt in fetal life, to close before birth (patent refers to remaining open) Polyvalvular disease – damage or defect to a heart valve (mitral, aortic, tricuspid or pulmonary); mitral and tricuspid valves control the flow of blood between the atria and the ventricles Pulmonary stenosis – narrowing or obstruction to blood flow (stenosis) from the right ventricle to the pulmonary artery Coarctation of aorta – narrowing of the aorta, the large blood vessel that delivers oxygen-rich blood to the body Bicuspid aortic valve - aortic valve separates the lower left chamber (left ventricle) of the heart from the aorta. A bicuspid aortic valve has two flaps (cusps) instead of the usual three. This condition is often present with coarctation of aorta. Mitral valve atresia – mitral valve connects the two chambers on the left side of the heart (atrium and ventricle). In this condition, blood is unable to flow between the two chambers. Hypoplastic aorta (hypoplastic left heart syndrome) – left side of the heart is critically underdeveloped so unable to effectively pump blood to the body and causing the right side of the heart to pump blood to the lungs and body. -

Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly
333 Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly A. James Barkovich 1 The MR scans of six pediatric patients with apparent atypical callosal dysgenesis (presence of the dorsal corpus callosum in the absence of a rostral corpus callosum) were critically analyzed and correlated with developmental information in order to assess the anatomic, embryologic, and developmental implications of this unusual anomaly. Four patients had semilobar holoprosencephaly; the dorsal interhemispheric commis sure in these four infants resembled a true callosal splenium. All patients in this group had severe developmental delay. The other two patients had complete callosal agenesis with an enlarged hippocampal commissure mimicking a callosal splenium; both were developmentally and neurologically normal. The embryologic implications of the pres ence of these atypical interhemispheric connections are discussed. Differentiation between semilobar holoprosencephaly and agenesis of the corpus callosum with enlarged hippocampal commissure-two types of apparent atypical callosal dysgenesis-can be made by obtaining coronal, short TR/TE MR images through the frontal lobes. Such differentiation has critical prognostic implications. AJNR 11:333-339, March{Apri11990 Abnormalities of the corpus callosum are frequently seen in patients with con genital brain malformations [1-5); a recent publication [5) reports an incidence of 47%. The corpus callosum normally develops in an anterior to posterior direction. The genu forms first, followed by the body, splenium, and rostrum. Dysgenesis of the corpus callosum is manifested by the presence of the earlier-formed segments (genu , body) and absence of the later-formed segments (splenium, rostrum) [4-6]. We have recently encountered six patients with findings suggestive of atypical callosal dysgenesis in whom there was apparent formation of the callosal splenium in the absence of the genu and body. -

Anatomy of the Corpus Callosum Reveals Its Function
The Journal of Neuroscience, February 13, 2008 • 28(7):1535–1536 • 1535 Journal Club Editor’s Note: These short critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and comentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml. Anatomy of the Corpus Callosum Reveals Its Function Eric Mooshagian Department of Psychology, University of California, Los Angeles, California 90095-1563 Review of Wahl et al. (http://www.jneurosci.org/cgi/content/full/27/45/12132) The corpus callosum (CC) comprises ax- views of the CC. In contrast, a few recent amining callosal topography, suggesting a ons connecting the cortices of the two ce- studies have used diffusion tensor imag- more posterior crossing of CMFs (for dis- rebral hemispheres and is the principal ing (DTI) methods to re-evaluate callosal cussion, see Wahl et al., 2007). In addi- white matter fiber bundle in the brain. As topography (for discussion, see Wahl et tion, the present study goes beyond a recently as the mid 20th century, the CC al., 2007). These methods challenge the demonstration of topography by reveal- was thought to serve no other purpose conventional partitioning schemes used ing, for the first time, a clear somatotopy than preventing the two hemispheres to divide the CC into functionally signifi- of CMFs; hand fibers were situated ventral from collapsing on one another (Bogen, cant regions (Witelson, 1989). and anterior to foot fibers, and lip fibers, 1979). -

Crossed Optic Ataxia: Possible Role of the Dorsal Splenium
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.46.6.533 on 1 June 1983. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1983;46:533-539 Crossed optic ataxia: possible role of the dorsal splenium JOSE M FERRO,* JM BRAVO-MARQUES,* A CASTRO-CALDAS,* LOBO ANTUNESt From the Language Research Laboratory* and the Neuropathology Laboratory, t Centro de Estudos Egas Moniz (INIC), Department ofNeurology, Hospital de Santa Maria, Lisbon, Portugal SUMMARY An unusual combination of disconnective syndromes is reported: transcortical motor aphasia, left arm apraxia and optic ataxia. Neuropathological examination showed a left parieto-occipital and a subcortical frontal infarct and a lesion of the dorsal part of the posterior two-fifths of the callosum. The frontal lesion caused the transcortical motor aphasia and pro- duced the left arm apraxia. Visuomotor incoordination in the right hemispace was due to the left parieto-occipital infarct, while the crossed optic ataxia in the left hemispace was attributed to the callosal lesion. It is proposed that the pathway that serves crossed visual reaching passes through the dorsal part of the posterior callosum. This case reinforces the growing evidence that fibres in the corpus callosum are arranged in ventro-dorsal functional lamination. Protected by copyright. Optic ataxia or visuomotor ataxia' consists of a dis- Recondo and Dumas', induces a crossed optic turbance in reaching under visual control that may ataxia, that is, inability to reach in one hemifield affect one or both hands, and be present either in with the hand of the opposite side) is, according to the whole or in part of the visual fields. -

A Pan-Mammalian Map of Interhemispheric Brain Connections Predates the Evolution of the Corpus Callosum
A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum Rodrigo Suáreza,1, Annalisa Paolinoa, Laura R. Fenlona, Laura R. Morcoma, Peter Kozulina, Nyoman D. Kurniawanb, and Linda J. Richardsa,c,1 aQueensland Brain Institute, The University of Queensland, Brisbane, QLD 4070, Australia; bCentre for Advanced Imaging, The University of Queensland, Brisbane, QLD 4070, Australia; and cSchool of Biomedical Sciences, The University of Queensland, Brisbane, QLD 4070, Australia Edited by Jon H. Kaas, Vanderbilt University, Nashville, TN, and approved August 1, 2018 (received for review May 14, 2018) The brain of mammals differs from that of all other vertebrates, in embryonic astroglia (13), which is exclusively present in euthe- having a six-layered neocortex that is extensively interconnected rians (14). The evolution of the corpus callosum as a distinct within and between hemispheres. Interhemispheric connections are tract allowed a significant expansion of the number of inter- conveyed through the anterior commissure in egg-laying mono- hemispheric neocortical connections in species with large brains tremes and marsupials, whereas eutherians evolved a separate (15). The corpus callosum carries fibers topographically arranged commissural tract, the corpus callosum. Although the pattern of according to the position of their cell bodies (16–18) and con- interhemispheric connectivity via the corpus callosum is broadly nects mostly similar (homotopic) but also different (heterotopic) shared across eutherian species, it is not known whether this pattern regions in each hemisphere (Fig. 1B). However, although the arose as a consequence of callosal evolution or instead corresponds map of callosal fibers in eutherians is well-established, and in- to a more ancient feature of mammalian brain organization. -

Corpus Callosum Deficiency in Transgenic Mice Expressing a Truncated Ephrin-A Receptor
The Journal of Neuroscience, November 26, 2003 • 23(34):10963–10970 • 10963 Development/Plasticity/Repair Corpus Callosum Deficiency in Transgenic Mice Expressing a Truncated Ephrin-A Receptor Zhaoliang Hu,1,3 Xin Yue,1,3 Guanfang Shi,1,3 Yong Yue,1,3 David P. Crockett,3 Jan Blair-Flynn,4 Kenneth Reuhl,2 Lino Tessarollo,4 and Renping Zhou1,3 1Laboratory for Cancer Research and 2Department of Pharmacology, College of Pharmacy, Rutgers University, and 3Department of Neuroscience and Cell Biology, Robert Wood Johnson Medical School, Piscataway, New Jersey 08854, and 4Neural Development Group, Mouse Cancer Genetics Program, National Cancer Institute–Frederick Cancer Research and Development Center, Frederick, Maryland 21701 The A-class of the erythropoietin-producing hepatocellular carcinoma cell-derived (EphA) tyrosine kinase receptors and their ligands, the A-ephrins, play critical roles in the specification of topographic axon projection maps during development. In this study, the role of the EphA subfamily in callosal projections was investigated using transgenic mice expressing a kinase deletion mutant of EphA5. In approximately half of these transgenic mice, cerebral cortical neurons in various cortical regions (primary and secondary somatosensory cortices and frontal as well as visual areas) failed to project to the contralateral cortex. When commissural axons were examined with DiI labeling, few callosal fibers were found to traverse the midline in both the adult and neonatal transgenic mice. This defect in callosal development correlates with the expression of the transgene, because neurons in the superficial layers of the motor cortex, where transgene expression is low, show normal contralateral projection through the corpus callosum. -

Central Glossary
33.3_9.1.10.qxd:32(1).qxp 9/8/10 11:44 AM Page 288 CENTRAL GLOSSARY Adaptive immune response: Second line of the immune response spleen. Blymphocytes produce antibodies. In people with that is specific to a given foreign molecule or pathogen and HIV, the ability of Blymphocytes to do their job may be leads to an “immunological memory” after the first response damaged. to the molecule or pathogen. Bacteremia: The presence of viable bacteria in the blood. Additive: The effect when two or more agents act together to Bronchiectasis: Disease of the airways characterized by a local achieve a result that is the sum of the effects achieved by the ized, irreversible widening (dilation) of a part of the airways; individual agents. the affected bronchi and bronchioles are dilated, inflamed AIDSdefining illness or event: Any one of a group of illnesses and collapse easily, resulting in reduced airflow; usually presence of which indicates that a patient’s HIV infection caused by bacterial infections. has progressed to the acquired immune deficiency syndrome Basal ganglia: A group of nuclei in the brain interconnected (AIDS); these conditions include, among others, yeast infections with the cerebral cortex, thalamus and brainstem. Mammalian of the airways (i.e., candidiasis), Kaposi’s sarcoma, certain basal ganglia are associated with a variety of functions: viral infections, tuberculosis, and certain cancers. motor control, cognition, emotions, and learning. Airway obstruction: Respiratory problem in which the small airways (bronchioles) show increased resistance, thereby Cachexia: A syndrome characterized by weight loss, muscle reducing the amount of air inhaled in each breath and, atrophy, fatigue, weakness, and loss of appetite that cannot consequently, the amount of oxygen that can be taken up be reversed by increased nutrition. -

Role of the Corpus Callosum in Functional Connectivity
AJNR Am J Neuroradiol 24:208–212, February 2003 Role of the Corpus Callosum in Functional Connectivity Michelle Quigley, Dietmar Cordes, Pat Turski, Chad Moritz, Victor Haughton, Raj Seth, and M. Elizabeth Meyerand BACKGROUND AND PURPOSE: Regional cerebral blood flow fluctuates synchronously in corresponding brain regions between the hemispheres. This synchrony implies neuronal con- nections between brain regions. The synchrony of blood flow changes is defined operationally as functional connectivity. Our purpose was to measure functional connectivity in patients with cor- pus callosal agenesis, in whom the interhemispheric connectivity is hypothetically diminished. METHODS: In three patients with agenesis of the corpus callosum, functional MR imaging was performed while patients performed text-listening and finger-tapping tasks. Functional images were also acquired while the patients performed no specific task (resting state). Regions of activation temporally correlated with the performance of the tasks were identified by cross-correlating the task data with a reference function. Voxel clusters (seed voxels) that corresponded to regions of activation in the task-activation data set were selected in the resting data set. All the voxels in the resting 3D data set that had a correlation coefficient exceeding 0.4 were identified. The number of these voxels in the ipsilateral and contralateral hemispheres was tabulated. RESULTS: In all patients, technically adequate functional MR and functional connectivity MR maps were obtained. For both tasks, activation was found in both hemispheres. For all of the seed voxels, significantly more functionally connected voxels were found in the ipsilateral hemisphere than in the contralateral hemisphere. For most seed voxels, no functionally connected voxels were found in the contralateral hemisphere. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
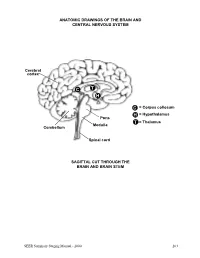
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -
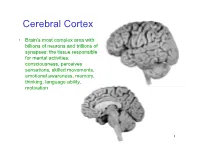
Cerebral Cortex
Cerebral Cortex • Brain’s most complex area with billions of neurons and trillions of synapses: the tissue responsible for mental activities: consciousness, perceives sensations, skilled movements, emotional awareness, memory, thinking, language ability, motivation 1 John Lorber, Science 210:1232 (1980) “There a young student at this university who has an IQ of 126, has gained a first-class honors degree in mathematics, and is socially completely normal. And yet the boy has virtually no brain.” “The student’s physician at the university noticed that the student had slightly larger than normal head… When we did a brain scan on him we saw that instead of the normal 4.5 cm thickness of brain tissue there was just a millimeter or so. His cranium is filled with CSF.” How is this possible? What does it tell us? Do you think this would be OK if it happened to an adult? To a 15 year old? To a 5 year old? To a neonate? 3 Types of Cerebral Cortex • Neocortex – Newest in evolution – About 90% of total – 6 layers, most complex • Paleocortex – Associated with olfactory system, the parahippocampal gyrus, uncus – fewer than 6 layers • Archicortex – Hippocampal formation; limbic system – 3 layers, most primitive • Mesocortex – Cingulate gyrus, insular cortex – Transitional between archicortex and neocortex 5 The perks of having a neocortex • The words used to describe the higher mental capacities of animals with a large neocortex include: – CONSCIOUSNESS – FREE WILL – INTELLIGENCE – INSIGHT • Animals with much simpler brains learn well, so LEARNING should not be among these capacities (Macphail 1982). • A species could have genetically determined mechanisms, acquired through evolutionary selection, for taking advantage of the regular features of the environment, or they could have learned through direct experience. -

Histochemical Changesin Neocortex and Corpus
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.32.4.317 on 1 August 1969. Downloaded from J. Neurol. Neurosurg. Psychiat., 1969, 32, 317-323 Histochemical changes in neocortex and corpus callosum after intracranial injection N. ROBINSON1 From the Department of Chemical Pathology, Institute ofNeurology, The National Hospital, Queen Square, London The penetration of a syringe needle or electrode into closed with silk suture material before the animals the brain, causing localized physical changes such recovered. In the second series of experiments the as a high compression front with displacement of microneedle was lowered into the corpus callosum at the whole cells and other cells suffering from various same surface co-ordinates as in the first series before in- jection of 5 ,ul. fluid. degrees of temporary distortion, would be expected The animals were killed at 1, 2, 3, 5, 7, 14, 21, and 31 to produce disturbances in metabolism. Subsequent days after operation. The brains were removed, rapidly intracranial injection of small quantities of fluid may frozen, and fresh-frozen sections 10 to 12 jL thick cut on guest. Protected by copyright. produce changes in pH, electrolyte concentration, a Slee cryostat. The techniques for demonstrating enzyme ionic strength, temperature, and the disequilibrium activities have been described (Robinson, 1969a); the of colloidal suspensions. Less clearly defined effects enzymes were NADH2-diaphorase, succinate, glucose on metabolism due to inflammatory processes and 6-phosphate and a-glycerophosphate dehydrogenases, trauma would also be likely to occur. 5'-nucleotidase, adenosine triphosphatase, thiamine pyro- This paper reports the effects of intracranial phosphatase, acid and alkaline phosphatase, acetyl- penetration of a microneedle and subsequent cholinesterase, and monoamine oxidase.