Uncorrected Pre-Publication Proof
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Creationist and the Sociobiologist: Two Stories About Illiberal Education
The Creationist and the Sociobiologist: Two Stories About Illiberal Education ILLIBERAL EDUCATION. By Dinesh D'Souza.t Reviewed by Phillip E. Johnsont There is a mad reductionism at work [in the universities]. God is not a proper topic for discussion, but "lesbian politics" is.... In the famous "marketplace of ideas," where all ideas are equal and where there must be no "value judgments" and therefore no values, certain ideas are simply excluded, and woe to those who espouse them.1 In a liberal culture, it is a great rhetorical advantage to appear in a dispute as the champion of free speech against the forces of repression. The left has held this advantage for a long time. The student revolt of the 1960s opened with a "Free Speech Movement," and the bumper sticker that directs us to "Question Authority" implies that the left's politics is a matter of raising questions rather than imposing answers. Recently, however, academic traditionalists like Dinesh D'Souza have seized the moral high ground by describing a left-imposed atmosphere of "political correctness" in the universities that leads to "illiberal educa- tion." In effect, they have captured the bumper sticker and turned its message around. The "PC left" under attack is post-Marxist, and its philosophy is post-Modernist. A brief pause for definitions is necessary. In post- Marxism, racial minorities, feminists, and gays have assumed the mantle of the proletariat; the oppressor class is heterosexist white males rather than the bourgeoisie; and the struggle is for control of the terms of dis- course rather than the means of production. -

Two Modern Compositional Approaches
Musical Aesthetics of the Natural World: Two Modern Compositional Approaches Eli Stine and Christopher Luna-Mega University of Virginia, McIntire Department of Music Throughout recorded human history, experiences and observations of the natural world have inspired the arts. Within the sonic arts, evocations of nature permeate a wide variety of acoustic and electronic composition strategies. These strategies artistically investigate diverse attributes of nature: tranquility, turbulence, abundance, scarcity, complexity, and purity, to name but a few. Within the 20th century, new technologies to understand these attributes, including media recording and scientific analysis, were developed. These technologies allow music composi- tion strategies to go beyond mere evocation and to allow for the construction of musical works that engage explicit models of nature (what has been called ‘biologically inspired music’). This paper explores two such deployments of these ‘natural sound models’ within music and music generation systems created by the authors: an electroacoustic composition using data derived from multi-channel recordings of forest insects (Luna-Mega) and an electronic music generation system that extracts musical events from the different layers of natural soundscapes, in particular oyster reef soundscapes (Stine). Together these works engage a diverse array of extra-musical disciplines: environmental science, acoustic ecology, entomology, and computer science. The works are contextualized with a brief history of natural sound models from pre- antiquity to the present in addition to reflections on the uses of technology within these projects and the potential experiences of audiences listening to these works. “Great art picks up where nature ends.” - Mark Chagall and aesthetics towards a more quantitative awareness of how The natural world has been a focal point for the arts since the natural world functions and evolves is present within a the Upper Paleolithic. -
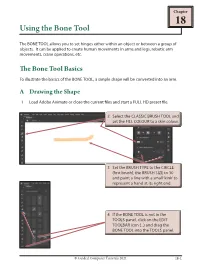
Using the Bone Tool
Chapter 18 Using the Bone Tool The BONE TOOL allows you to set hinges either within an object or between a group of objects. It can be applied to create human movements in arms and legs, robotic arm movements, crane operations, etc. The Bone Tool Basics To illustrate the basics of the BONE TOOL, a simple shape will be converted into an arm. A Drawing the Shape 1 Load Adobe Animate or close the current files and start a FULL HD preset file. © Guided Computer Tutorials 2021 18-1 Learning Adobe Animate CC B Applying the Bone Tool 1 Press CTRL+ or COMMAND+ to zoom the view to 200%. 3 When you release the mouse button the first bone is created. NOTE: This first section will represent a bone from the shoulder to the elbow. 5 Release the mouse button to create the second bone. NOTE: This second section will represent a bone from the elbow to the wrist. 18-2 © Guided Computer Tutorials 2021 Using the Bone Tool 18 NOTE: All the bone sections are moved into the ARMATURE layer. C Using the Bone Links The bone sections have set a rotation joint at the left of the shape (red diamond shape) and hinges (or joints) at the centre and near the right of the shape. 1 Press CTRL- or COMMAND- to return the view to 100%. NOTE: When the mouse pointer is over a joint or bone that can be moved, a bone symbol is added to the pointer. © Guided Computer Tutorials 2021 18-3 Learning Adobe Animate CC D The Pin Option The PIN option allows you to fix the position of a bone and prevent it from moving. -

Adobe Animate Cc Classroom in a Book (2018 Release) 301
NATURAL AND CHARACTER 9 ANIMATION Lesson Overview In this lesson, you’ll learn how to do the following: • Use the Bone tool to build armatures (skeletons) of movie clips • Use the Bone tool to build armatures of shapes • Animate natural motion of armatures using inverse kinematics • Constrain and pin the armature joints • Edit the position of armature bones and joints • Refine shape deformations with the Bind tool • Simulate physics with the Spring feature • Adjust the speed setting to add a sense of weight to armatures This lesson will take about one and a half hours to complete. Please log in to your account on peachpit.com to download the lesson files for this chap- ter, or go to the Getting Started section at the beginning of this book and follow the instructions under “Accessing the Lesson Files and Web Edition.” 298 From the Library of Alvaro Alvarez You can easily create complex and natural motion with articulations—joints between linked objects and within shapes—by using the Bone tool for animation in a process called inverse kinematics . 299 From the Library of Alvaro Alvarez Getting Started You’ll start the lesson by viewing the animated walking monkey that you’ll create as you learn about natural motion in Adobe Animate CC. 1 Double-click the 09End.html file in the Lesson09/09End folder to play the animation. The animation depicts a cartoon monkey walking in an endless cycle with a scrolling motion in the background. His arms and legs swing naturally, and his tail curls and unfurls naturally and smoothly. -

Language Evolution to Revolution: from a Slowly Developing Finite Communication System with Many Words to Infinite Modern Language
bioRxiv preprint doi: https://doi.org/10.1101/166520; this version posted July 20, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Language evolution to revolution: from a slowly developing finite communication system with many words to infinite modern language Andrey Vyshedskiy1,2* 1Boston University, Boston, USA 2ImagiRation LLC, Boston, MA, USA Keywords: Language evolution, hominin evolution, human evolution, recursive language, flexible syntax, human language, syntactic language, modern language, Cognitive revolution, Great Leap Forward, Upper Paleolithic Revolution, Neanderthal language Abstract There is overwhelming archeological and genetic evidence that modern speech apparatus was acquired by hominins by 600,000 years ago. There is also widespread agreement that modern syntactic language arose with behavioral modernity around 100,000 years ago. We attempted to answer two crucial questions: (1) how different was the communication system of hominins before acquisition of modern language and (2) what triggered the acquisition of modern language 100,000 years ago. We conclude that the communication system of hominins prior to 100,000 years ago was finite and not- recursive. It may have had thousands of words but was lacking flexible syntax, spatial prepositions, verb tenses, and other features that enable modern human language to communicate an infinite number of ideas. We argue that a synergistic confluence of a genetic mutation that dramatically slowed down the prefrontal cortex (PFC) development in monozygotic twins and their spontaneous invention of spatial prepositions 100,000 years ago resulted in acquisition of PFC-driven constructive imagination (mental synthesis) and converted the finite communication system of their ancestors into infinite modern language. -

Homo Erectus Infancy and Childhood the Turning Point in the Evolution of Behavioral Development in Hominids
10 Homo erectus Infancy and Childhood The Turning Point in the Evolution of Behavioral Development in Hominids Sue Taylor Parker In man, attachment is mediated by several different sorts of behaviour of which the most obvious are crying and calling, babbling and smiling, clinging, non-nutritional sucking, and locomotion as used in approach, following and seeking. —John Bowlby, Attachment The evolution of hominid behavioral ontogeny can be recon - structed using two lines of evidence: first, comparative neontological data on the behavior and development of living hominoid species (humans and the great apes), and second, comparative paleontolog- ical and archaeological evidence associated with fossil hominids. (Although behavior rarely fossilizes, it can leave significant traces.) 1 In this chapter I focus on paleontological and neontological evi - dence relevant to modeling the evolution of the following hominid adaptations: (1) bipedal locomotion and stance; (2) tool use and tool making; (3) subsistence patterns; (4) growth and development and other life history patterns; (5) childbirth; (6) childhood and child care; and (7) cognition and cognitive development. In each case I present a cladistic model for the origins of the characters in question. 2 Specifically, I review pertinent data on the following widely recog - nized hominid genera and species: Australopithecus species (A. afarensis , A. africanus , and A. robustus [Paranthropus robustus]) , early Homo species (Australopithecus gahri , Homo habilis , and Homo rudolfensis) , and Middle Pleistocene Homo species (Homo erectus , Homo ergaster , and others), which I am calling erectines . Copyrighted Material www.sarpress.org 279 S UE TAYLOR PARKER Table 10.1 Estimated Body Weights and Geological Ages of Fossil Hominids _______________________________________________________________________ Species Geologic Age Male Weight Female Weight (MYA) (kg) (kg) _______________________________________________________________________ A. -

Neither Chimpanzee Nor Human, Ardipithecus Reveals the Surprising Ancestry of Both Tim D
SPECIAL FEATURE: PERSPECTIVE PERSPECTIVE SPECIAL FEATURE: Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both Tim D. Whitea,1, C. Owen Lovejoyb, Berhane Asfawc, Joshua P. Carlsona, and Gen Suwad,1 aDepartment of Integrative Biology, Human Evolution Research Center, University of California, Berkeley, CA 94720; bDepartment of Anthropology, School of Biomedical Sciences, Kent State University, Kent, OH 44242–0001; cRift Valley Research Service, Addis Ababa, Ethiopia; and dThe University Museum, The University of Tokyo, Hongo, Bunkyo-ku Tokyo 113-0033, Japan Edited by Neil H. Shubin, University of Chicago, Chicago, IL, and approved September 10, 2014 (received for review April 25, 2014) Australopithecus fossils were regularly interpreted during the late 20th century in a framework that used living African apes, especially chimpanzees, as proxies for the immediate ancestors of the human clade. Such projection is now largely nullified by the discovery of Ardipithecus. In the context of accumulating evidence from genetics, developmental biology, anatomy, ecology, biogeography, and geology, Ardipithecus alters perspectives on how our earliest hominid ancestors—and our closest living relatives—evolved. human evolution | Australopithecus | hominid | Ethiopia “...the stock whence two or more species have chimpanzees, can serve as adequate repre- (5). Indeed, a widely used textbook still pro- sprung, need in no respect be intermediate sentations of the ancestral past. claims that, “Overall, Au. afarensis seems very between those species.” much like a missing link between the living Background T. H. Huxley, 1860 (1) Africanapesandlaterhomininsinitsdental, ’ Darwin s human evolution scenario attemp- cranial, and skeletal morphology” (6). Charles Darwin famously suggested that ted to explain hominid tool use, bipedality, Australopithecus can no longer be legiti- Africa was humanity’s most probable birth enlarged brains, and reduced canine teeth (2). -
How Did Language Begin?
How did language begin? Written by Ray Jackendoff What does the question mean? In asking about the origins of human language, we first have to make clear what the question is. The question is not how languages gradually developed over time into the languages of the world today. Rather, it is how the human species developed over time so that we — and not our closest relatives, the chimpanzees and bonobos — became capable of using language. And what an amazing development this was! No other natural communication system is like human language. Human language can express thoughts on an unlimited number of topics (the weather, the war, the past, the future, mathematics, gossip, fairy tales, how to fix the sink...). It can be used not just to convey information, but to solicit information (ques- tions) and to give orders. Unlike any other animal communication system, it contains an expression for negation — what is not the case. Every human lan- guage has a vocabulary of tens of thousands of words, built up from several dozen speech sounds. Speakers can build an unlimited number of phrases and sentences out of words plus a smallish collec- tion of prefixes and suffixes, and the meanings of sentences are built from the meanings of the individ- ual words. What is still more remarkable is that every normal child learns the whole system from hearing others use it. Animal communication systems, in contrast, typically have at most a few dozen distinct calls, and they are used only to communicate immediate issues such as food, danger, threat, or reconciliation. -

Bibliography
Bibliography Many books were read and researched in the compilation of Binford, L. R, 1983, Working at Archaeology. Academic Press, The Encyclopedic Dictionary of Archaeology: New York. Binford, L. R, and Binford, S. R (eds.), 1968, New Perspectives in American Museum of Natural History, 1993, The First Humans. Archaeology. Aldine, Chicago. HarperSanFrancisco, San Francisco. Braidwood, R 1.,1960, Archaeologists and What They Do. Franklin American Museum of Natural History, 1993, People of the Stone Watts, New York. Age. HarperSanFrancisco, San Francisco. Branigan, Keith (ed.), 1982, The Atlas ofArchaeology. St. Martin's, American Museum of Natural History, 1994, New World and Pacific New York. Civilizations. HarperSanFrancisco, San Francisco. Bray, w., and Tump, D., 1972, Penguin Dictionary ofArchaeology. American Museum of Natural History, 1994, Old World Civiliza Penguin, New York. tions. HarperSanFrancisco, San Francisco. Brennan, L., 1973, Beginner's Guide to Archaeology. Stackpole Ashmore, w., and Sharer, R. J., 1988, Discovering Our Past: A Brief Books, Harrisburg, PA. Introduction to Archaeology. Mayfield, Mountain View, CA. Broderick, M., and Morton, A. A., 1924, A Concise Dictionary of Atkinson, R J. C., 1985, Field Archaeology, 2d ed. Hyperion, New Egyptian Archaeology. Ares Publishers, Chicago. York. Brothwell, D., 1963, Digging Up Bones: The Excavation, Treatment Bacon, E. (ed.), 1976, The Great Archaeologists. Bobbs-Merrill, and Study ofHuman Skeletal Remains. British Museum, London. New York. Brothwell, D., and Higgs, E. (eds.), 1969, Science in Archaeology, Bahn, P., 1993, Collins Dictionary of Archaeology. ABC-CLIO, 2d ed. Thames and Hudson, London. Santa Barbara, CA. Budge, E. A. Wallis, 1929, The Rosetta Stone. Dover, New York. Bahn, P. -

Homo Heidelbergensis: the Ot Ol to Our Success Alexander Burkard Virginia Commonwealth University
Virginia Commonwealth University VCU Scholars Compass Auctus: The ourJ nal of Undergraduate Research and Creative Scholarship 2016 Homo heidelbergensis: The oT ol to Our Success Alexander Burkard Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/auctus Part of the Archaeological Anthropology Commons, Biological and Physical Anthropology Commons, and the Biology Commons © The Author(s) Downloaded from https://scholarscompass.vcu.edu/auctus/47 This Social Sciences is brought to you for free and open access by VCU Scholars Compass. It has been accepted for inclusion in Auctus: The ourJ nal of Undergraduate Research and Creative Scholarship by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Homo heidelbergensis: The Tool to Our Success By Alexander Burkard Homo heidelbergensis, a physiological variant of the species Homo sapien, is an extinct spe- cies that existed in both Europe and parts of Asia from 700,000 years ago to roughly 300,000 years ago (carbon dating). This “subspecies” of Homo sapiens, as it is formally classified, is a direct ancestor of anatomically modern humans, and is understood to have many of the same physiological characteristics as those of anatomically modern humans while still expressing many of the same physiological attributes of Homo erectus, an earlier human ancestor. Since Homo heidelbergensis represents attributes of both species, it has therefore earned the classifica- tion as a subspecies of Homo sapiens and Homo erectus. Homo heidelbergensis, like anatomically modern humans, is the byproduct of millions of years of natural selection and genetic variation. It is understood through current scientific theory that roughly 200,000 years ago (carbon dat- ing), archaic Homo sapiens and Homo erectus left Africa in pursuit of the small and large animal game that were migrating north into Europe and Asia. -

Mechanics of Bipedalism: an Exploration of Skeletal Morphology and Force Plate Anaylsis Erin Forse May 04, 2007 a Senior Thesis
MECHANICS OF BIPEDALISM: AN EXPLORATION OF SKELETAL MORPHOLOGY AND FORCE PLATE ANAYLSIS ERIN FORSE MAY 04, 2007 A SENIOR THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF BACHELOR OF ARTS IN ARCHAEOLOGICAL STUDIES UNIVERSITY OF WISCONSIN- LA CROSSE Abstract There are several theories on how humans learned to walk, and while these all address the adaptations needed for walking, none adequately describes how our early ancestors developed the mechanism to walk. Our earliest recognizable relatives, the australopithecines, have several variations on a theme: walking upright. There are varied changes as australopithecines approach the genus Homo. These changes occurred in the spine, legs, pelvis, and feet, and changes are also in the cranium, arms and hands, but these are features that may have occurred simultaneously with bipedalism. Several analyses of Australopithecus afarensis, specifically specimen A.L. 288-1 ("Lucy"), have shown that the skeletal changes are intermediate between apes and humans. Force plate analyses are used to determine if the gait pattern of humans resembles that of apes, and if it is a likely development pattern. The results of both these analyses will give insight into how modern humans developed bipedalism. Introduction Bipedalism is classified as movement of the post-cranial body in a vertical position, with the lower limbs shifting as an inverted pendulum, progressing forward. Simply, it is upright walking. Several theories have addressed why bipedalism evolved in hominids, with some unlikely ideas taking hold throughout the history of the issue. Other theories are more likely, but all lack the same characteristic: answering how bipedalism developed. -

NEW ARCHAEOLOGICAL PUBLICATIONS in GEORGIA VAKHTANG LICHELI Otar Lortqifanize, Zveli Qartuli Civilizaciis Sataveebtan
ACSS_F8_314-322 1/10/07 3:14 PM Page 315 NEW ARCHAEOLOGICAL PUBLICATIONS IN GEORGIA VAKHTANG LICHELI Otar Lortqifanize, Zveli qartuli civilizaciis sataveebtan (The Sources of Ancient Georgian Civilization). Tbilisi, State University of Tbilisi, 2002, 338 pages, ISBN 99928-931-3-3 (In Georgian) This book by the well-known Georgian archaeologist, who founded the Centre for Archaeological Research of the Georgian Academy of Sciences, Academician Otar Lordkipanidze, is a third revised and extended edition. The Russian version was published as far back as 1989 in Tbilisi under the title The Heritage of Ancient Georgia. The next edition appeared in Germany in German as Archäologie in Georgien. Von der Altsteinzeit zum Mittelalter (Weinheim, VCH, Acta Humaniora) in 1991. The Georgian edition has been revised and extended: archaeological mater- ial has been added and it now contains a review of the scholarly literature up to the year 2000. The book consists of an introduction and four chapters, illus- trations and – what is particularly important – an extended bibliography with 1717 titles and a virtually complete list of publications on Georgian archaeol- ogy in Georgian, Russian and other languages. Apart from the research which occupies the main part of the book, this additional section is particularly valu- able, since O. Lordkipanidze had been the first to collect and analyze works on all questions concerning Georgian archaeology. The introduction examines questions relating to the origin and ethno- linguistic history of the Georgians, written sources in Georgian and other lan- guages; it also presents the reader with toponymic, onomastic, linguistic, ethnographic and archaeological data and information drawn from myths and folklore.