Vinculin, and A-Actinin K.-L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wiskott-Aldrich Syndrome: the Actin Cytoskeleton and Immune Cell Function
Disease Markers 29 (2010) 157–175 157 DOI 10.3233/DMA-2010-0735 IOS Press The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function Michael P. Blundella, Austen Wortha,b, Gerben Boumaa and Adrian J. Thrashera,b,∗ aMolecular Immunology Unit, UCL Institute of Child Health, London, UK bDepartment of Immunology, Great Ormond Street Hospital NHS Trust, Great Ormond Street, London, UK Abstract. Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive primary immunodeficiency characterised by immune dysregulation, microthrombocytopaenia, eczema and lymphoid malignancies. Mutations in the WAS gene can lead to distinct syndrome variations which largely, although not exclusively, depend upon the mutation. Premature termination and deletions abrogate Wiskott-Aldrich syndrome protein (WASp) expression and lead to severe disease (WAS). Missense mutations usually result in reduced protein expression and the phenotypically milder X-linked thrombocytopenia (XLT) or attenuated WAS [1–3]. More recently however novel activating mutations have been described that give rise to X-linked neutropenia (XLN), a third syndrome defined by neutropenia with variable myelodysplasia [4–6]. WASP is key in transducing signals from the cell surface to the actin cytoskeleton, and a lack of WASp results in cytoskeletal defects that compromise multiple aspects of normal cellular activity including proliferation, phagocytosis, immune synapse formation, adhesion and directed migration. Keywords: Wiskott-Aldrich syndrome, actin polymerization, lymphocytes, -

Desmin Forms Toxic, Seeding-Competent Amyloid Aggregates That Persist in Muscle Fibers
Desmin forms toxic, seeding-competent amyloid aggregates that persist in muscle fibers Niraja Kediaa, Khalid Arhzaouyb, Sara K. Pittmanb, Yuanzi Sunc,d, Mark Batchelord, Conrad C. Weihlb,1, and Jan Bieschkea,d,1 aDepartment of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63130; bDepartment of Neurology, Washington University School of Medicine, St. Louis, MO 63110; cDepartment of Energy, Environmental and Chemical Engineering, Washington University in St. Louis, St. Louis, MO 63130; and dUniversity College London Institute of Prion Diseases/Medical Research Council Prion Unit, University College London, London W1W 7FF, United Kingdom Edited by Nancy M. Bonini, University of Pennsylvania, Philadelphia, PA, and approved July 10, 2019 (received for review May 16, 2019) Desmin-associated myofibrillar myopathy (MFM) has pathologic Desmin is a 470-amino acid protein, and more than 70 disease- similarities to neurodegeneration-associated protein aggregate dis- associated mutations have been reported that span the entire eases. Desmin is an abundant muscle-specific intermediate filament, protein (3). The formation of desmin IFs occurs via sequentially and disease mutations lead to its aggregation in cells, animals, and ordered steps that include dimer and tetramer formation, unit- patients. We reasoned that similar to neurodegeneration-associated length filament formation, and filament elongation (5). Some proteins, desmin itself may form amyloid. Desmin peptides corre- disease mutations affect IF assembly in vitro and in vivo, resulting sponding to putative amyloidogenic regions formed seeding- in cytosolic inclusions (5, 6). Similarly, disease mutations in the competent amyloid fibrils. Amyloid formation was increased when small heat shock protein αB crystallin affect its ability to facili- disease-associated mutations were made within the peptide, and tate desmin filament formation, resulting in desmin aggregation this conversion was inhibited by the anti-amyloid compound (7). -

Vinculin Antibody Cat
Vinculin Antibody Cat. No.: 7809 Vinculin Antibody Figure 2 Western Blot Validation in Human, Mouse and Rat Cell Lines Figure 3 Western Blot Validation in PC3 Cell Lysate Loading: 15 μg of lysates per lane. Antibodies: Vinculin Loading: 15 μg of lysates per lane. Antibodies: Vinculin 7809 (1 μg/mL), 1h incubation at RT in 5% NFDM/TBST. 7809 (Lane 1: 0.5 μg/mL and Lane 2: 1 μg/mL), 1h Secondary: Goat anti-rabbit IgG HRP conjugate at 1:10000 incubation at RT in 5% NFDM/TBST. Secondary: Goat anti- dilution. rabbit IgG HRP conjugate at 1:10000 dilution. Figure 4 Immunocytochemistry Validation of Vinculin in Jurkat Cells Figure 5 Immunofluorescence Validation of Vinculin in Immunocytochemical analysis of Jurkat cells using anti- Jurkat Cells Vinculin antibody (7809) at 5 μg/ml. Cells was fixed with Immunofluorescent analysis of 4% paraformaldehyde- formaldehyde and blocked with 10% serum for 1 h at RT; fixed Jurkat cells labeling Vinculin with 7809 at 20 μg/mL, antigen retrieval was by heat mediation with a citrate followed by goat anti-rabbit IgG secondary antibody at buffer (pH6). Samples were incubated with primary 1/500 dilution (green) and DAPI staining (blue). antibody overnight at 4˚C. A goat anti-rabbit IgG H&L (HRP) at 1/250 was used as secondary. Counter stained with Hematoxylin. Specifications September 23, 2021 1 https://www.prosci-inc.com/vinculin-antibody-7809.html HOST SPECIES: Rabbit SPECIES REACTIVITY: Human, Mouse, Rat HOMOLOGY: Predicted species reactivity based on immunogen sequence: Pig: (100%) Anti-Vinculin antibody (7809) was raised against a peptide corresponding to 23 amino IMMUNOGEN: acids near the center of human Vinculin. -

How Microtubules Control Focal Adhesion Dynamics
JCB: Review Targeting and transport: How microtubules control focal adhesion dynamics Samantha Stehbens and Torsten Wittmann Department of Cell and Tissue Biology, University of California, San Francisco, San Francisco, CA 94143 Directional cell migration requires force generation that of integrin-mediated, nascent adhesions near the cell’s leading relies on the coordinated remodeling of interactions with edge, which either rapidly turn over or connect to the actin cytoskeleton (Parsons et al., 2010). Actomyosin-mediated the extracellular matrix (ECM), which is mediated by pulling forces allow a subset of these nascent FAs to grow integrin-based focal adhesions (FAs). Normal FA turn- and mature, and provide forward traction forces. However, in over requires dynamic microtubules, and three members order for cells to productively move forward, FAs also have to of the diverse group of microtubule plus-end-tracking release and disassemble underneath the cell body and in the proteins are principally involved in mediating micro- rear of the cell. Spatial and temporal control of turnover of tubule interactions with FAs. Microtubules also alter these mature FAs is important, as they provide a counterbalance to forward traction forces, and regulated FA disassembly is the assembly state of FAs by modulating Rho GTPase required for forward translocation of the cell body. An important signaling, and recent evidence suggests that microtubule- question that we are only beginning to understand is how FA mediated clathrin-dependent and -independent endo turnover is spatially and temporally regulated to allow cells cytosis regulates FA dynamics. In addition, FA-associated to appropriately respond to extracellular signals, allowing for microtubules may provide a polarized microtubule track for coordinated and productive movement. -
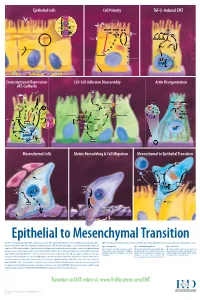
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Daam2 Couples Translocation and Clustering of Wnt Receptor Signalosomes Through Rac1 Carlo D
© 2021. Published by The Company of Biologists Ltd | Journal of Cell Science (2021) 134, jcs251140. doi:10.1242/jcs.251140 RESEARCH ARTICLE Daam2 couples translocation and clustering of Wnt receptor signalosomes through Rac1 Carlo D. Cristobal1,QiYe2, Juyeon Jo2, Xiaoyun Ding3, Chih-Yen Wang2, Diego Cortes2, Zheng Chen4 and Hyun Kyoung Lee1,3,5,* ABSTRACT Dynamic polymerization of the Dishevelled proteins functions at Wnt signaling plays a critical role in development across species and the core of the Wnt signalosome by interacting with both the is dysregulated in a host of human diseases. A key step in signal Frizzled Wnt receptors and low-density lipoprotein receptor-related transduction is the formation of Wnt receptor signalosomes, during protein 5/6 (LRP5/6), leading to recruitment of Axin proteins β which a large number of components translocate to the membrane, from the -catenin destruction complex (MacDonald et al., 2009; cluster together and amplify downstream signaling. However, the Schwarz-Romond et al., 2007). However, the exact composition molecular processes that coordinate these events remain poorly and mechanisms of signalosome assembly at the plasma membrane defined. Here, we show that Daam2 regulates canonical Wnt remain unclear. signaling via the PIP –PIP5K axis through its association with Rac1. The hallmark of canonical Wnt signaling is the accumulation and 2 β Clustering of Daam2-mediated Wnt receptor complexes requires both translocation of -catenin into the nucleus for gene transcription, β Rac1 and PIP5K, and PIP5K promotes membrane localization of these whereas non-canonical Wnt signaling is -catenin independent and complexes in a Rac1-dependent manner. Importantly, the localization involves assembly/disassembly of the actin cytoskeleton, polarized of Daam2 complexes and Daam2-mediated canonical Wnt signaling is cell shape changes and cell migration (Niehrs, 2012; Schlessinger dependent upon actin polymerization. -

Actin-Bound Structures of Wiskott–Aldrich Syndrome Protein (WASP)-Homology Domain 2 and the Implications for Filament Assembly
Actin-bound structures of Wiskott–Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly David Chereau, Frederic Kerff, Philip Graceffa, Zenon Grabarek, Knut Langsetmo, and Roberto Dominguez* Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02472 Edited by Thomas D. Pollard, Yale University, New Haven, CT, and approved September 28, 2005 (received for review August 12, 2005) Wiskott–Aldrich syndrome protein (WASP)-homology domain 2 It has been proposed, based on sequence analysis, that WH2 (WH2) is a small and widespread actin-binding motif. In the WASP forms part of an extended family with the thymosin  domain family, WH2 plays a role in filament nucleation by Arp2͞3 complex. (T) (7). However, this view is controversial, in part because of Here we describe the crystal structures of complexes of actin with the different biological functions and low sequence similarity of the WH2 domains of WASP, WASP-family verprolin homologous WH2 and T (8). The actin-bound structures of the N-terminal protein, and WASP-interacting protein. Despite low sequence iden- half of ciboulot domain 1 (9) and that of a hybrid protein tity, WH2 shares structural similarity with the N-terminal portion of consisting of gelsolin domain 1 and the C-terminal half of T4 the actin monomer-sequestering thymosin  domain (T). We (10) have been reported. These structures have been combined show that both domains inhibit nucleotide exchange by targeting into a model of T4–actin (10), and, although both T4 and the cleft between actin subdomains 1 and 3, a common binding site ciboulot belong in the T family, their structures have been for many unrelated actin-binding proteins. -

Catenin Controls Vinculin Binding
ARTICLE Received 4 Feb 2014 | Accepted 25 Jun 2014 | Published 31 Jul 2014 DOI: 10.1038/ncomms5525 Force-dependent conformational switch of a-catenin controls vinculin binding Mingxi Yao1,*, Wu Qiu2,3,*, Ruchuan Liu2,3, Artem K. Efremov1, Peiwen Cong1,4, Rima Seddiki5, Manon Payre5, Chwee Teck Lim1,6, Benoit Ladoux1,5,Rene´-Marc Me`ge5 & Jie Yan1,3,6,7 Force sensing at cadherin-mediated adhesions is critical for their proper function. a-Catenin, which links cadherins to actomyosin, has a crucial role in this mechanosensing process. It has been hypothesized that force promotes vinculin binding, although this has never been demonstrated. X-ray structure further suggests that a-catenin adopts a stable auto-inhibitory conformation that makes the vinculin-binding site inaccessible. Here, by stretching single a- catenin molecules using magnetic tweezers, we show that the subdomains MI vinculin- binding domain (VBD) to MIII unfold in three characteristic steps: a reversible step at B5pN and two non-equilibrium steps at 10–15 pN. 5 pN unfolding forces trigger vinculin binding to the MI domain in a 1:1 ratio with nanomolar affinity, preventing MI domain refolding after force is released. Our findings demonstrate that physiologically relevant forces reversibly unfurl a- catenin, activating vinculin binding, which then stabilizes a-catenin in its open conformation, transforming force into a sustainable biochemical signal. 1 Mechanobiology Institute, National University of Singapore, Singapore 117411, Singapore. 2 Department of Physics, National University of Singapore, Singapore 117542, Singapore. 3 College of Physics, Chongqing University, No. 55 Daxuecheng South Road, Chongqing 401331, China. 4 Singapore-MIT Alliance for Research and Technology, National University of Singapore, Singapore 117543, Singapore. -

The Ubiquitin-Proteasome Pathway Mediates Gelsolin Protein Downregulation in Pancreatic Cancer
The Ubiquitin-Proteasome Pathway Mediates Gelsolin Protein Downregulation in Pancreatic Cancer Xiao-Guang Ni,1 Lu Zhou,2 Gui-Qi Wang,1 Shang-Mei Liu,3 Xiao-Feng Bai,4 Fang Liu,5 Maikel P Peppelenbosch,2 and Ping Zhao4 1Department of Endoscopy, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; 2Department of Cell Biology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; 3Department of Pathology, 4Department of Abdominal Surgery, and 5State Key Laboratory of Molecular Oncology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China A well-known observation with respect to cancer biology is that transformed cells display a disturbed cytoskeleton. The under- lying mechanisms, however, remain only partly understood. In an effort to identify possible mechanisms, we compared the pro- teome of pancreatic cancer with matched normal pancreas and observed diminished protein levels of gelsolin—an actin fila- ment severing and capping protein of crucial importance for maintaining cytoskeletal integrity—in pancreatic cancer. Additionally, pancreatic ductal adenocarcinomas displayed substantially decreased levels of gelsolin as judged by Western blot and immunohistochemical analyses of tissue micoarrays, when compared with cancerous and untransformed tissue from the same patients (P < 0.05). Importantly, no marked downregulation of gelsolin mRNA was observed (P > 0.05), suggesting that post- transcriptional mechanisms mediate low gelsolin protein levels. In apparent agreement, high activity ubiquitin-proteasome path- way in both patient samples and the BxPC-3 pancreatic cancer cell line was detected, and inhibition of the 26s proteasome sys- tem quickly restored gelsolin protein levels in the latter cell line. -

A Unique and Specific Interaction Between Αt-Catenin and Plakophilin
2126 Research Article A unique and specific interaction between ␣T-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs Steven Goossens1,2,*, Barbara Janssens1,2,*,‡, Stefan Bonné1,2,§, Riet De Rycke1,2, Filip Braet1,2,¶, Jolanda van Hengel1,2 and Frans van Roy1,2,** 1Department for Molecular Biomedical Research, VIB and 2Department of Molecular Biology, Ghent University, B-9052 Ghent, Belgium *These authors contributed equally to this work ‡Present address: Wiley-VCH, Boschstrasse 12, D-69469 Weinheim, Germany §Present address: Diabetes Research Center, Brussels Free University (VUB), Laarbeeklaan 103, B-1090 Brussels, Belgium ¶Present address: Australian Key Centre for Microscopy and Microanalysis, The University of Sydney, NSW 2006, Australia **Author for correspondence (e-mail: [email protected]) Accepted 24 April 2007 Journal of Cell Science 120, 2126-2136 Published by The Company of Biologists 2007 doi:10.1242/jcs.004713 Summary Alpha-catenins play key functional roles in cadherin- desmosomal proteins in the heart localize not only to the catenin cell-cell adhesion complexes. We previously desmosomes in the intercalated discs but also at adhering reported on ␣T-catenin, a novel member of the ␣-catenin junctions with hybrid composition. We found that in the protein family. ␣T-catenin is expressed predominantly in latter junctions, endogenous plakophilin-2 colocalizes with cardiomyocytes, where it colocalizes with ␣E-catenin at the ␣T-catenin. By providing an extra link between the intercalated discs. Whether ␣T- and ␣E-catenin have cadherin-catenin complex and intermediate filaments, the specific or synergistic functions remains unknown. In this binding of ␣T-catenin to plakophilin-2 is proposed to be a study we used the yeast two-hybrid approach to identify means of modulating and strengthening cell-cell adhesion specific functions of ␣T-catenin. -

A Computational Study of Stress Fiber-Focal Adhesion Dynamics
A computational study of stress fiber-focal adhesion dynamics governing cell contractility M. Maraldi1, C. Valero2, K. Garikipati1;3∗ 1Department of Mechanical Engineering, University of Michigan, Ann Arbor, Michigan 2M2BE, Aragon´ Institute of Engineering Research (I3A), University of Zaragoza, Zaragoza, Spain 3Department of Mathematics, University of Michigan, Ann Arbor, Michigan Abstract We apply a recently developed model of cytoskeletal force generation to study a cell’s intrinsic contractility, as well as its response to external loading. The model is based on a non-equilibrium thermodynamic treatment of the mechano-chemistry governing force in the stress fiber-focal adhesion system. Our computational study suggests that the mechan- ical coupling between the stress fibers and focal adhesions leads to a complex, dynamic, mechano-chemical response. We collect the results in response maps whose regimes are distinguished by the initial geometry of the stress fiber-focal adhesion system, and by the external load on the cell. The results from our model connect qualitatively with recent stud- ies on the force response of smooth muscle cells on arrays of polymeric microposts (Mann et al., Lab. on a Chip, 12, 731-740, 2012). INTRODUCTION In contractile cells, such as smooth muscle cells and fibroblasts, the generation of traction force is the result of two different actions: myosin-powered cytoskeletal contractility and external mechanical stimuli (applied stretch or force). The cooperation between these two aspects de- termines the level of the force within the cell and influences the development of cytoskeletal components via the (un)binding of proteins. Important cytoskeletal components that mediate this interplay of mechanics and chemistry are stress fibers and focal adhesions. -

The Role of the Interaction of the Vinculin Proline-Rich Linker Region
ß 2014. Published by The Company of Biologists Ltd | Journal of Cell Science (2014) 127, 1875–1886 doi:10.1242/jcs.133645 RESEARCH ARTICLE The role of the interaction of the vinculin proline-rich linker region with vinexin a in sensing the stiffness of the extracellular matrix Hiroshi Yamashita1,*, Takafumi Ichikawa1,*, Daisuke Matsuyama1, Yasuhisa Kimura1, Kazumitsu Ueda1,2, Susan W. Craig3, Ichiro Harada4 and Noriyuki Kioka1,` ABSTRACT information into biochemical signals. At FAs, transmembrane ECM receptors, integrins, are connected to the ends of actin stress Although extracellular matrix (ECM) stiffness is an important aspect fibers through various cytoplasmic proteins, including talin and of the extracellular microenvironment and is known to direct the vinculin. Cells sense ECM stiffness by ‘pulling’ on the ECM lineage specification of stem cells and affect cancer progression, using actomyosin-generated intracellular tension through FAs and the molecular mechanisms that sense ECM stiffness have not yet adjusting the tension that they exert accordingly (Geiger et al., been elucidated. In this study, we show that the proline-rich linker 2009; Parsons et al., 2010); cells on rigid ECM exert higher (PRL) region of vinculin and the PRL-region-binding protein vinexin intracellular tension than cells on soft ECM. Although some FA are involved in sensing the stiffness of ECM substrates. A rigid proteins have been suggested to engage in sensing intracellular substrate increases the level of cytoskeleton-associated vinculin, tension (del Rio et al., 2009; Friedland et al., 2009; Sawada et al., and the fraction of vinculin stably localizing at focal adhesions (FAs) 2006), the signaling mechanisms by which cells sense ECM is larger on rigid ECM than on soft ECM.