Role of Androgen and Estrogen Receptors for the Action of Dehydroepiandrosterone (DHEA)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Anatomia Associada Ao Comportamento Reprodutivo De
Jimena García Rodríguez Anatomia associada ao comportamento reprodutivo de Cubozoa Anatomy associated with the reproductive behavior of Cubozoa São Paulo 2015 Jimena García Rodríguez Anatomia associada ao comportamento reprodutivo de Cubozoa Anatomy associated with the reproductive behavior of Cubozoa Dissertação apresentada ao Instituto de Biociências da Universidade de São Paulo para obtenção de Título de Mestre em Ciências, na Área de Zoologia Orientador: Prof. Dr. Antonio Carlos Marques São Paulo 2015 García Rodríguez, Jimena Anatomia associada ao comportamento reprodutivo de Cubozoa 96 páginas Dissertação (Mestrado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Zoologia. 1. Cubozoa; 2. Histologia; 3. Reprodução. I. Universidade de São Paulo. Instituto de Biociências. Departamento de Zoologia. Comissão Julgadora Prof(a) Dr(a) Prof(a) Dr(a) Prof. Dr. Antonio Carlos Marques A mis padres, hermana y en especial a mi abuelita “Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. Al andar se hace el camino, y al volver la vista atrás se ve la senda que nunca se ha de volver a pisar. Caminante no hay camino sino estelas en la mar” Antonio Machado, 1912 Agradecimentos Em primeiro lugar, eu gostaria de agradecer ao meu orientador Antonio Carlos Marques, Tim, pela confiança desde o primeiro dia, pela ajuda tanto pessoal como profissional durante os dois anos de mestrado, pelas discussões de cada tema tratado e estudado e pelas orientações que tornaram possível a elaboração deste trabalho. Agradeço também o apoio institucional do Instituto de Biociências e do Centro de Biologia Marinha da Universidade de São Paulo. -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Earthworms (Clitellata, Acanthodrilidae) of the Mountains of Eastern Jamaica Samuel W
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector ARTICLE IN PRESS Organisms, Diversity & Evolution 4 (2004) 277–294 www.elsevier.de/ode Earthworms (Clitellata, Acanthodrilidae) of the mountains of Eastern Jamaica Samuel W. James University of Kansas Natural History Museum and Biodiversity Research Center, Dyche Hall, 1345 Jayhawk Drive, Lawrence, KS 66045, USA Received 20 May 2003; accepted 20 April 2004 Abstract Fourteen species new to science are described from material collected at several sites in the Blue Mountains and the John Crow Mountains of eastern Jamaica, doubling the known endemic Jamaican earthworm fauna. New data on Dichogaster montecyanensis (Sims) are provided. All species are placed in the genus Dichogaster Beddard, which is here treated sensu lato, i.e. including Eutrigaster Cognetti. Eight of the new species have lost the posterior pair of prostates and the seminal grooves of the male field. These are D. bromeliocola, D. crossleyi, D. davidi, D. garciai, D. harperi, D. haruvi, D. hendrixi, and D. johnsoni. D. sydneyi n. sp. has independently lost the posterior prostates but not the seminal grooves. The new species D. altissima and D. manleyi have the conventional dichogastrine prostatic battery and male field characteristics. Three species described here, D. farri, D. garrawayi, and D. marleyi, all have a third pair of prostates in the 20th segment, no seminal grooves, dorsal paired intestinal caeca in segment lxv, and lack penial setae. r 2004 Elsevier GmbH. All rights reserved. Keywords: Dichogaster; Oligochaeta; Clitellata; Jamaica; Earthworms Introduction It being my intent to find the native earthworms of Jamaica, I did not concern myself with making a Scattered over the last century one can find four complete inventory of all earthworms present, including primary sources on the earthworm fauna of Jamaica— exotics. -

The Male Body
Fact Sheet The Male Body What is the male What is the epididymis? reproductive system? The epididymis is a thin highly coiled tube (duct) A man’s fertility and sexual characteristics depend that lies at the back of each testis and connects on the normal functioning of the male reproductive the seminiferous tubules in the testis to another system. A number of individual organs act single tube called the vas deferens. together to make up the male reproductive 1 system; some are visible, such as the penis and the 6 scrotum, whereas some are hidden within the body. The brain also has an important role in controlling 7 12 reproductive function. 2 8 1 11 What are the testes? 3 6 The testes (testis: singular) are a pair of egg 9 7 12 shaped glands that sit in the scrotum next to the 2 8 base of the penis on the outside of the body. In 4 10 11 adult men, each testis is normally between 15 and 3 35 mL in volume. The testes are needed for the 5 male reproductive system to function normally. 9 The testes have two related but separate roles: 4 10 • to make sperm 5 1 Bladder • to make testosterone. 2 Vas deferens The testes develop inside the abdomen in the 3 Urethra male fetus and then move down (descend) into the scrotum before or just after birth. The descent 4 Penis of the testes is important for fertility as a cooler 5 Scrotum temperature is needed to make sperm and for 16 BladderSeminal vesicle normal testicular function. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Treating Localized Prostate Cancer
Treating Localized Prostate Cancer A Review of the Research for Adults Is this information right for me? Yes, this information is right for you if: Your doctor* said all tests show you have localized prostate cancer (the cancer has not spread outside the prostate gland). This information may not be helpful to you if: Your prostate cancer has spread to other parts of your body. What will this summary tell me? This summary will tell you about: What localized prostate cancer is Common treatment options for localized prostate cancer (watchful waiting, active surveillance, surgery to remove the prostate gland, radiation therapy, and hormone treatment) What researchers found about how the treatments compare Possible side effects of the treatments Things to talk about with your doctor This summary does not cover: How to prevent prostate cancer Less common treatments for localized prostate cancer, such as high-intensity focused ultrasound (high-energy sound waves), cryotherapy (freezing treatment), proton-beam radiation therapy (radiation with a beam of protons instead of x-rays), and stereotactic body radiation therapy (high-energy focused radiation) Herbal products or vitamins and minerals Treatments (such as chemotherapy) for cancer that has spread outside the prostate gland * In this summary, the term doctor refers to your health care professional, including your primary care physician, urologist, oncologist, nurse practitioner, or physician assistant. What is the source of the information? Researchers funded by the Agency for Healthcare Research and Quality, a Federal Government research agency, reviewed studies on treatments for localized prostate cancer published between January 1, 2007, and March 7, 2014. -
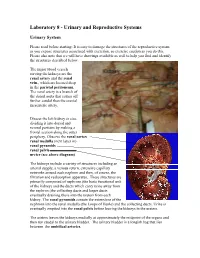
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Animal / Dairy Science 434 Testis Comparative Male Anatomy Species Testis Wt
Animal / Dairy Science 434 Testis Comparative Male Anatomy Species Testis Wt. % Of Orientation (grams) Body Wt. bull 300+ .03 vertical ram 250 .3 vertical boar 350 .2 oblique stallion 200 .03 horizontal dog 25 .2 oblique/horz. cat --- --- oblique rabbit 2.5 .1 vertical human 25 .03 vertical Reproductive Organs of the Stallion Seminal Vesicles Prostate Rectum Reproductive Cowpers Seminal Vesicles Ampulla Gland Prostate Organs of the Ampulla Rectum Cowper’s Bull Bladder Bladder Gland Vas Deferens Retractor Vas Deferens Penis Retractor Glans Muscle Corpus Cavernosum Penis Penis Muscle Sigmoid Gubernaculum Flexure Cauda Caput Epididymis Caput Testis Scrotum Glans Penis Prepuce Scrotum Testis Cauda Epididymis Prepuce Gubernaculum Reproductive Organs of the Ram Bull Vertical Vertical Anatomy of the Dog Boar Oblique Oblique to Horizontal Stallion Human Vertical Horizontal Testis Orientation Testis Species Testis Wt. % Of Orientation Bull (grams) Body Wt. Tail Head bull 300+ .03 vertical ram 250 .3 vertical boar 350 .2 oblique stallion 200 .03 horizontal dog 25 .2 oblique/horz. cat --- --- oblique rabbit 2.5 .1 vertical human 25 .03 vertical The Stallion Pelvic Genitalia of the Stallion Bladder Seminal Cowper’s Body of Cowper’s Vesicles Gland Prostate Gland Bulbospongiosus Seminal Bulbospongiosus Muscle Vesicles Urethra Muscle Vas Ampulla Deferens Ampulla Crus Body of Penis Prostate Retractor Colliculus Seminalis Penis Muscle Pelvic Urethral Ischiocavernosus No Disseminate Muscle Vas Prostate Pelvic Urethral Muscle Deferens Muscle Seminal Ram -

Prostatitis Crónica: Una Revisión Crítica De Su Actual Definición Nosológica, Clasificación Y Potencial Carcinogénesis
Artículo Especial Arch. Esp. Urol., 60, 6 (617-623), 2007 PROSTATITIS CRÓNICA: UNA REVISIÓN CRÍTICA DE SU ACTUAL DEFINICIÓN NOSOLÓGICA, CLASIFICACIÓN Y POTENCIAL CARCINOGÉNESIS. Remigio Vela Navarrete, Carmen González Enguita, Juan Vicente García Cardoso, G. Manzarbeitia y F. Soriano García. Cátedra de Urología de la Universidad Autónoma de Madrid. Servicio de Urología de la Fundación Jiménez Díaz. Departamentos de Anatomía Patológica y Microbiología de la Fundación Jiménez Díaz. Madrid. España. Resumen.- Revisión crítica actualizada de la prostati- Palabras clave: Prostatitis crónica. Inflamación tis crónica, como entidad nosológica, anatomoclínica, prostática. Dolor pélvico. supuestamente de origen microbiológico o inflamatorio. Argumentación científica, a la luz de los nuevos pro- gresos, sobre el papel de la inflamación amicrobiana, Summary.- Updated critical review of chronic prosta- tanto a nivel de la próstata craneal como de la caudal, titis as a nosologic, anatomic-clinical entity of supposed para reconsiderar la conveniencia de mantener la cla- microbiological or inflammatory origin. Scientific reaso- sificación actual de las prostatitis crónicas, y especial- ning about the role of amicrobial inflammation in both mente el apartado referido a la “prostatitis histológica”. caudal and cranial prostate, after new progresses, to Análisis de evidencias relacionando la prostatitis “con el reconsider the convenience of maintaining the current dolor pélvico”, síndrome dominante en muchos pacien- classification of chronic prostatitis, mainly in the section tes y fundamento de la actual propuesta terminológica; referred to “histological prostatitis”. Analysis of scientific prostatitis-dolor pélvico. Papel de la inflamación en la evidences relating prostatitis and “pelvic pain”, the do- génesis de la HBP y cáncer de próstata. Justificación y minant syndrome in many patients and basement of the conveniencia de un nuevo consenso terminológico so- current terminological proposal: prostatitis-pelvic pain.