New Insights on the Effects of Formulation Type and Compositional Mixtures Over
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agrimonia Eupatoria L. and Cynara Cardunculus L. Water Infusions: Phenolic Profile and Comparison of Antioxidant Activities
Article Agrimonia eupatoria L. and Cynara cardunculus L. Water Infusions: Phenolic Profile and Comparison of Antioxidant Activities Anika Kuczmannová 1,†, Peter Gál 1,2,3,4,†,*, Lenka Varinská 2,3, Jakub Treml 5, Ivan Kováˇc 3,6, Martin Novotný 3,7, Tomáš Vasilenko 3,8, Stefano Dall’Acqua 9, Milan Nagy 1 and Pavel Muˇcaji 1,* Received: 29 September 2015 ; Accepted: 4 November 2015 ; Published: 18 November 2015 Academic Editor: Maurizio Battino 1 Department of Pharmacognosy and Botany, Faculty of Pharmacy, Comenius University, Odbojárov 10, 832 32 Bratislava, Slovakia; [email protected] (A.K.); [email protected] (M.N.) 2 Department of Pharmacology, Faculty of Medicine, Pavol Jozef Šafárik University, Trieda SNP 1, 040 11 Košice, Slovakia; [email protected] 3 Department for Biomedical Research, East-Slovak Institute of Cardiovascular Diseases, Inc., Ondavská 8, 040 11 Košice, Slovakia; [email protected] (I.K.); [email protected] (M.N.); [email protected] (T.V.) 4 Institute of Anatomy, 1st Faculty of Medicine, Charles University, U nemocnice 2, 128 00 Prague, Czech Republic 5 Department of Molecular Biology and Pharmaceutical Biotechnology, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Palackého 1-3, 612 42 Brno, Czech Republic; [email protected] 6 2nd Department of Surgery, Pavol Jozef Šafárik University and Louise Pasteur University Hospital, 041 90 Košice, Slovakia 7 Department of Infectology and Travel Medicine, Pavol Jozef Šafárik University and Louise Pasteur University Hospital, 041 90 Košice, Slovakia 8 Department of Surgery, Pavol Jozef Šafárik University and Košice-Šaca Hospital, 040 15 Košice-Šaca, Slovakia 9 Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Via F. -

Bhuaamla Or Bhumyamalaki Has Been Used in Ayurveda, Siddha for Problems of the Stomach, Genito Urinary System, Liver, Kidney, and Spleen, and to Treat Chronic Fever
KEVA D-TOXIPLUS Liver is an important organ of the body There are over 100 different problems in liver in the form of diseases that affect men, women and children Any defect in liver causes weakness and fatigue, weight loss, nausea, vomiting, and jaundice There are many kinds of liver diseases Diseases caused by viruses • HEPATITIS A • HEPATITIS B • HEPATITIS C liver diseases Diseases caused by drugs, poisons, or too much alcohol FATTY LIVER DISEASE CIRRHOSIS liver diseases Liver cancer Inherited diseases Hemochromatosis Wilson disease Reasons for liver problems Excessive amounts of acetaminophen Alcohol Abuse Hepatitis A, B, C, D and E Epstein Barr virus (infectious mononucleosis) Iron Overload Alcoholism (Hemochromatosis) With the help of powerful botanical blend D-ToxiPlus helps in nourishing and protecting the liver so that it can do its job well Can provide therapeutic benefits in Liver diseases and disorders Helps in normalizing the functioning of Liver Cells as it has natural antioxidant properties that may support liver cell integrity Detoxification is an essential step for achieving and maintaining good health and to rejuvenate the entire body at a cellular level. Detoxification should be done regularly to improve the functioning of vital organs like the liver and kidneys and to overcome chronic diseases This is a NATURAL & SAFE PRODUCT recommended to be used for all age groups Ingredients Cochlospermum Chelidonium Majus Angolensis Rumex Crispus Phyllantus Niruri Linum Usita Cichorium Intybus tissimim L Taraxacum Officinale Silybum Marianum Cochlospermum Angolensis Borututu (Cochlospermum angolensis) is an African tree whose bark has recently emerged as a herbal dietary supplement with claims for antioxidant activity. -

Botanical Authentication of Globe Artichoke-Containing Foods: Differentiation of Cynara Scolymus by a Novel HRM Approach
Food Chemistry 366 (2022) 130621 Contents lists available at ScienceDirect Food Chemistry journal homepage: www.elsevier.com/locate/foodchem Botanical authentication of globe artichoke-containing foods: Differentiation of Cynara scolymus by a novel HRM approach Liliana Grazina a, Andreia Batista a, Joana S. Amaral b, Joana Costa a, Isabel Mafra a,* a REQUIMTE-LAQV, Faculdade de Farmacia,´ Universidade do Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal b Centro de Investigaçao~ de Montanha (CIMO), Instituto Polit´ecnico de Bragança, Campus de Sta. Apolonia,´ 5301-857 Bragança, Portugal ARTICLE INFO ABSTRACT Keywords: Cynara scolymus L., known as globe artichoke, is a medicinal plant widely used in plant food supplements (PFS) Globe artichoke and herbal infusions due to its beneficial health properties. The high demand for artichoke-containing products Plant food supplements can lead to adulteration practices. In this work, a real-time polymerase chain reaction (PCR) system coupled to Authenticity high-resolution melting (HRM) analysis was proposed to differentiate C. scolymus from other Cynara species. Real-time PCR Hence, a Cynara-specific real-time PCR assay was successfully developed with high analytical performance, High-resolution melting analysis achieving a sensitivity of 0.4 pg of globe artichoke DNA. HRM analysis enabled the discrimination of C. scolymus, with a high level of confidence (>98%), corroborating sequencing data. Application results to artichoke- containing PFS and mixed herbal infusions allowed confirming the presence of C. scolymus in 38% of the sam ples, suggesting the substitution/mislabelling of globe artichoke in 2 samples and the need for further efforts to increase DNA amplifiability of PFS. -

Spices, Herbs, and Such
Spices, Herbs, and Such Mark Hassman / June 2013 Abacateiro Leaf Bear Root Buckthorne Bark Chrysanthemum Flower Abuto Bedstraw Herb Bugleweed Chuchuhuasi Bark Acacia Flower Bee Pollen Bupleurum Root Cichona Bark Acanthopanax Beet Root Burdock Leaf Cilantro Acerola Bergamot Leaf Burdock Root Cinnamon Korinjte Achiote Leaf Betel Nut Butchers Broom Granules Achyranthes Root Beth Root Butcher's Broom Root Cinnamon Powder Aconite Root Bilberry Leaf/Fruit Butea Flower Cinnamon Sticks Adzuki Bean Birch Bark Butterbur Leaf/Root Cinquefoil Agar Agar Powder Bistort Button Snake Root Cistanche Powder Agaricus Mushroom Bittersweet Stalks Cactus Root/Leaf/Flower Citric Acid Agrimony Blackberry Leaf Calamus Root Sweet Clary Sage Ajamoda Power Black Catechu Flag Clay Bentonite Ajowan Seek Black Cohosh Calcatrippe Larkspur Clay French Green Akebia Vine Black Cohosh Root Calendula Marigold Clay Kaolin White Alfalfa Leaf / Seed Black Currant Leaf/Bark Camphor Crystals Clay Moroccan Red Alkanet Root Black Haw Bark Camu Camu Berry Cleavers Herb Allspice Black Henna Caraway Seed Clove Bud Aloe Cape Black Horehound Cardamon Seed Club Moss Aloe Vera Powder Black Radish Root Carline Thistle Root Cnidium Fruit Alteris Root Black Walnut Carob Bean Codonopsis Powder Alum Bark/Leaf/Hull Cascara Sagrada Coleus Root Amalaki Fruit Bladderwrack Cassie Bark Colombo Root Anamu Herb Blessed Thistle Catnip Coltsfoot Andrographis Blood Root Cat's Claw Bark Combretum Angelica Root Blueberry Leaf Catuaba Bark Comfrey Leaf Anise Seed Blue Cohosh Cayenne Powder Comfrey Root Anise -

Malva Neglecta Wallr.) Based on Color, Organic Acids, Total Phenolics and Antioxidant Parameters
molecules Article Quality Control of Gamma Irradiated Dwarf Mallow (Malva neglecta Wallr.) Based on Color, Organic Acids, Total Phenolics and Antioxidant Parameters José Pinela 1,2, Lillian Barros 1, Amilcar L. Antonio 1,3, Ana Maria Carvalho 1, M. Beatriz P. P. Oliveira 2 and Isabel C. F. R. Ferreira 1,* 1 Mountain Research Centre (CIMO), ESA, Polytechnic Institute of Bragança, Campus de Santa Apolónia, 1172, 5300-253 Bragança, Portugal; [email protected] (J.P.); [email protected] (L.B.); [email protected] (A.L.A.); [email protected] (A.M.C.) 2 REQUIMTE/LAQV, Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira, no. 228, 4050-313 Porto, Portugal; [email protected] 3 Centro de Ciências e Tecnologias Nucleares (C2TN), Instituto Superior Técnico, Universidade de Lisboa, E.N. 10, 2695-066 Bobadela, Portugal * Correspondence: [email protected]; Tel.: +351-273-303-219; Fax: +351-273-325-405 Academic Editor: Derek J. McPhee Received: 7 March 2016; Accepted: 7 April 2016; Published: 8 April 2016 Abstract: This study addresses the effects of gamma irradiation (1, 5 and 8 kGy) on color, organic acids, total phenolics, total flavonoids, and antioxidant activity of dwarf mallow (Malva neglecta Wallr.). Organic acids were analyzed by ultra fast liquid chromatography (UFLC) coupled to a photodiode array (PDA) detector. Total phenolics and flavonoids were measured by the Folin-Ciocalteu and aluminium chloride colorimetric methods, respectively. The antioxidant activity was evaluated based on the DPPH‚ scavenging activity, reducing power, β-carotene bleaching inhibition and thiobarbituric acid reactive substances (TBARS) formation inhibition. Analyses were performed in the non-irradiated and irradiated plant material, as well as in decoctions obtained from the same samples. -

Effects of Gamma Radiation on Chemical and Antioxidant Properties, Anti-Hepatocellular Carcinoma Activity and Hepatotoxicity Of
Effects of gamma radiation on chemical and antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of borututu Carla Pereiraa, Ricardo C. Calhelhaa,b, Amilcar L. Antonioa,c, Maria João R.P. Queirozb, Lillian Barrosa,*, Isabel C.F.R. Ferreiraa,* aCentro de Investigação de Montanha (CIMO), ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, apartado 1172, 5301-855 Bragança, Portugal bCentro de Química, Universidade do Minho, Campus de Gualtar 4710-057 Braga, Portugal cCentro de Ciências e Tecnologias Nucleares, Universidade de Lisboa, Estrada Nacional 10, 2686-953 Sacavém, Portugal * Authors to whom correspondence should be addressed (e-mail: [email protected] telephone +351-273-303219; fax +351-273-325405; email: [email protected]; telephone +351-273-303200; fax +351-273-325405). ABSTRACT Borututu is a well-known medicinal plant in Angola for the treatment of liver diseases and for the prophylaxis of malaria. Our research group reported, in a recent study, that its infusion, pills and syrups display significant antioxidant and anti-hepatocellular carcinoma activities. However, during the processing and storage, it can be easily exposed to contamination that can lead to a microbial deterioration or insect infestation compromising its quality, shelf life, and efficiency. Herein, we investigated the effect of gamma irradiation, one of the most promising decontamination methods for many foodstuffs and plant materials, at different doses (1 and 10 kGy) on borututu regarding to its nutritional value, lipophilic (fatty acids and tocopherols) and hydrophilic (free sugars and organic acids) compounds, antioxidant and anti-hepatocellular carcinoma activities. In general, the irradiation treatment did not appreciably affect the nutritional value of the studied plant, but the highest energetic contribution (383.83 kcal/100 g), total sugars (8.63 g/100 g), organic acids (3.31 g/100 g dw), total tocopherols (336.72 mg/100 g dw), and PUFA (32.75%) contents were found in the sample irradiated at 10 kGy. -

Hepatoprotective Activity of Medicinal Plants: a Mini Review
Journal of Medicinal Plants Studies 2020; 8(5): 183-188 ISSN (E): 2320-3862 ISSN (P): 2394-0530 Hepatoprotective activity of medicinal plants: A NAAS Rating: 3.53 www.plantsjournal.com mini review JMPS 2020; 8(5): 183-188 © 2020 JMPS Received: 09-07-2020 Accepted: 13-08-2020 Divya Jain, Priya Chaudhary, Aarti Kotnala, Rajib Hossain, Kiran Bisht and Mohammad Nabil Hossain Divya Jain Department of Bioscience and Biotechnology, Banasthali DOI: https://doi.org/10.22271/plants.2020.v8.i5c.1212 Vidyapith, Rajasthan, India Abstract Priya Chaudhary A phytotherapeutic approach for the development of modern drug agents can provide various vital drugs Department of Bioscience and from the conventional medicinal plants. Exploration of pure phytocompounds as drug agent is expensive Biotechnology, Banasthali and time taken. Various plants and their herbal formulations are found to be useful for the cure of liver Vidyapith, Rajasthan, India diseases. However, in some cases, the results of treatment are not up to the mark but experimental studies are carried out on a number of valuable plants and their formulations. The therapeutic efficacy is tested Aarti Kotnala against some chemically induced liver damages in liver cancer cell lines and animal models. Azoth Biotech LLP, Gautam Antioxidants from the medicinal plants and common dietary sources can provide protection against the Buddha Nagar, Noida, Uttar liver damages caused by the chemicals and oxidative stress mechanism. Pradesh, India Keywords: Antioxidants, liver diseases, liver cancer cell line, herbal formulation, hepatoprotective, Rajib Hossain phytotherapeutic Department of Pharmacy, Life Science Faculty, Bangabandhu Sheikh Mujibur Rahman Science Introduction and Technology University, In severe cases of liver damage, most of the cells either die or changes to fibrotic state. -

Infusions from Thymus Vulgaris L. Treated at Different Gamma Radiation Doses: Effects on Antioxidant Activity and Phenolic Compo
Infusions from Thymus vulgaris L. treated at different gamma radiation doses: effects on antioxidant activity and phenolic composition Running title: Gamma irradiation effects on antioxidants of thyme infusions Eliana Pereiraa,b, Lillian Barrosa,c, Amilcar L. Antonioa,d, Sandra Cabo Verded, Celestino Santos-Buelgab, Isabel C.F.R. Ferreiraa,* aCentro de Investigação de Montanha (CIMO), ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, 1172, 5300-253 Bragança, Portugal. bGIP-USAL, Facultad de Farmacia, Universidad de Salamanca, Campus Miguel de Unamuno, 37007 Salamanca, Spain. cLaboratory of Separation and Reaction Engineering - Laboratory of Catalysis and Materials (LSRE-LCM), Polytechnic Institute of Bragança, Campus de Santa Apolónia, 1134, 5301-857 Bragança, Portugal. dCentro de Ciências e Tecnologias Nucleares (C2TN), Instituto Superior Técnico, Universidade de Lisboa, E.N. 10, 2695-066 Bobadela, Portugal. *Author to whom correspondence should be addressed (e-mail: [email protected], telephone +351273303219, fax +351273325405). 1 ABSTRACT The use of ionizing radiation dates back to many years ago, and is accredited for application in different foods with several purposes. It has been increasingly used in many countries for the treatment of aromatic plants. Thymus vulgaris L. (thyme) is a plant commonly used by food, pharmaceutical and cosmetic industries representing a natural source of several bioactives such as phenolic compounds. The aim of this work was to evaluate the effects of gamma radiation on the antioxidant -

114Th Annual Meeting of the American Associaton of Colleges of Pharmacy, Chicago, IL, July 13-17, 2013
American Journal of Pharmaceutical Education 2013; 77 (5) Article 109. MEETING ABSTRACTS 114th Annual Meeting of the American Associaton of Colleges of Pharmacy, Chicago, IL, July 13-17, 2013 RESEARCH/EDUCATION ABSTRACTS grades in the aforementioned courses. Results: PAT scores, entering Biological Sciences GPA and composite PCAT scores positively correlated with the final grades for A&P, P.Chem, and Pharmaceutics. The PAT and the com- Completed Research: posite PCAT showed comparable correlations to the course grades. While the entering GPA showed a positive albeit a weak correlation A Novel Continuing Pharmacy Education Program: Overcoming with course grades. There was also a strong correlation between the Barriers to Healthcare in Transgender Patients. Kevin Eich, St. John final grades of each of the first-year courses. Implications: Success in Fisher College, Bernard P. Ricca, St. John Fisher College,Keith these first year courses often predict how well a student will perform DelMonte, St. John Fisher College, Jennifer L. Mathews, St. John in subsequent years of the program. Therefore, the PAT may serve as Fisher College. Objectives: The Wegmans School of Pharmacy strives an additional indicator of success rate in the first year. Furthermore, the to educate and expose students to many topics related to cultural com- PAT may help identify potential incoming students who may require petence, including transgender health. A continuing pharmacy educa- additional academic support and remediation opportunities to success- tion program (CPE) was utilized to promote a similar opportunity for fully complete the program in a timely manner. pharmacists and preceptors. The CPE aimed to educate those currently in practice so they may model the behavior which is reflective of the An Educational Board Game to Assist in Learning Autonomic school’s mission of providing appropriate care and services to all Nervous System Pharmacology. -
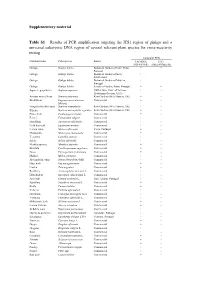
Results of PCR Amplification Targeting the ITS1 Region of Ginkgo and a Universal Eukaryotic
Supplementary material Table S1 – Results of PCR amplification targeting the ITS1 region of ginkgo and a universal eukaryotic DNA region of several relevant plant species for cross-reactivity testing End-point PCR Common name Plant species Source 18S rRNA ITS1 (EG-F/EG-R) (Gkb2-F/Gkb2-R) Ginkgo Ginkgo biloba Botanical Garden of Univ. Porto, + + Portugal Ginkgo Ginkgo biloba Botanical Garden of Bern, + + Switzerland Ginkgo Ginkgo biloba Botanical Garden of Madeira, + + Portugal Ginkgo Ginkgo biloba Serralves Garden, Porto, Portugal + + Japanese pagoda tree Sophora japonica USDA Grin, Univ. of Arizona + - Herbarium (Tucson, USA) Arizona mescal bean Sophora arizonica Kew Gardens (West Sussex, UK) + - Buckwheat Fagopyrum esculentum Commercial + - Moench Fringeleaf necklacepod Sophora stenophylla Kew Gardens (West Sussex, UK) + - Kōwhai Sophora microphylla x godleyi Kew Gardens (West Sussex, UK) + - Prince herb Cymbopogon citratus Commercial + - Fennel Foeniculum vulgare Commercial + - Dandelion Taraxacum officinales Commercial + - Field horsetail Equisetum arvense Commercial + - Lemon balm Melissa officinalis Viseu, Portugal + - Chamomile Matricaria chamomilla Commercial + - Tea plant Camellia sinensis Commercial + - Salvia Salvia officinalis Commercial + - Mentha piperita Mentha x piperita Commercial + - Borututu Cochlospermum angolense Commercial + - Gorse Pterospartum tridentatum Commercial + - Mallow Malva sylvestris Commercial + - Alexandrian senna Senna alexandrina Mill Commercial + - Mate herb Ilex paraguariensis Commercial + - Linden -
Wild Plants, Mushrooms and Nuts Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications
Wild Plants, Mushrooms and Nuts Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications Edited by Isabel C. F. R. Ferreira, Patricia Morales, and Lillian Barros Mountain Research Centre (CIMO), School of Agriculture, Polytechnic Institute of Bragança, Portugal Department of Nutrition and Bromatology II, Faculty of Pharmacy, Complutense University of Madrid, Spain Mountain Research Centre (CIMO), School of Agriculture, Polytechnic Institute of Bragança, Portugal This edition first published 2017 © 2017 John Wiley & Sons, Ltd Registered Office John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom For details of our global editorial offices, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com. The right of the author to be identified as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. Designations used by companies to distinguish their products are often claimed as trademarks. All brand names and product names used in this book are trade names, service marks, trademarks or registered trademarks of their respective owners. -

Milk Thistle, Myrrh and Mint: Herbal Plants As Natural Medicines Mohamad Hesam Shahrajabian1*, Wenli Sun1, Qi Cheng1,2
Nutrition and Food Sciences Research Vol 8, No 3, Jul-Sep 2021,pages: 59-65 Review Article Milk Thistle, Myrrh and Mint: Herbal Plants as Natural Medicines Mohamad Hesam Shahrajabian1*, Wenli Sun1, Qi Cheng1,2 1-Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China 2-College of Life Sciences, Hebei Agricultural University, Baoding, Hebei, 071000, China; Global Alliance of HeBAU-CLS&HeQiS for BioAl- Manufacturing, Baoding, Hebei 071000, China Received: November 2020 Accepted: February 2021 A B S T R A C T Herbs are natural products and herbal medicine has become a popular form of healthcare. Herbal-derived remedies may increase pharmacological qualities and improve prevention and treatment of several diseases. In this study, all relevant articles in English language were collected. Keywords of milk thistle, myrrh, mint and natural products were searched in Google Scholar, Scopus, Research Gate and PubMed databases. Milk thistile is a valuable widely-consumed botanical used for its various health benefits. The plant is an annual herb, belonging to Asteraceae family, which ripe seeds contain flavonoid substances. Myrrh is a sap-like substance, which is released from cuts in the barks of trees. The plant is a member of Commiphora genus. Mentha spicata is a species of mint native to Europe and South-East Asia. Mint is a creeping rhizomatous and perennial herb. In this mini-review, key roles and pharmaceutical benefits of milk thistle, myrrh and mint are described. Keywords: Milk thistle, Myrrh, Mint,