Bosutinib-Related Pneumonitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bosutinib (Bosulif®) (“Boe SUE Ti Nib”)
Bosutinib (Bosulif®) (“boe SUE ti nib”) How drug is given: By mouth Purpose: To stop the growth of cancer cells in chronic myelogenous leukemia (CML). How to take this drug 1. Take this medication with food. 2. Swallow each tablet whole; do not crush or chew. IF you have trouble swallowing the tablet, the pharmacist will give you specific instructions. 3. IF you miss a dose, take it as soon as possible. However, if it is less than 12 hours until your next dose, skip the missed dose and go back to your regular dosing schedule. Do not double dose. 4. Wash hands after taking the medication. Avoid handling crushed or broken tablets. 5. Do not drink grapefruit juice or eat grapefruit. Also, do not take St. John’s wort. Consuming these may increase the amount of medication in your body and worsen side effects. 6. Bosutinib can interFere with many drugs, which may change how this works in your body. Talk with your doctor before starting any new drugs, including over the counter drugs, natural products, herbals or vitamins. This includes drugs such as Prilosec™. Storage • Store this medication at room temperature 68-77°F (20-25°C), away From heat, moisture, and direct light. Keep this medicine in its original container, out oF reach oF children and pets. Things that may occur during treatment 1. Loose stools or diarrhea may occur within a few days after the drug is started. You may take loperamide (Imodium A-D®) to help control diarrhea. You may buy this at most drug stores. -

The Effects of Combination Treatments on Drug Resistance in Chronic Myeloid Leukaemia: an Evaluation of the Tyrosine Kinase Inhibitors Axitinib and Asciminib H
Lindström and Friedman BMC Cancer (2020) 20:397 https://doi.org/10.1186/s12885-020-06782-9 RESEARCH ARTICLE Open Access The effects of combination treatments on drug resistance in chronic myeloid leukaemia: an evaluation of the tyrosine kinase inhibitors axitinib and asciminib H. Jonathan G. Lindström and Ran Friedman* Abstract Background: Chronic myeloid leukaemia is in principle a treatable malignancy but drug resistance is lowering survival. Recent drug discoveries have opened up new options for drug combinations, which is a concept used in other areas for preventing drug resistance. Two of these are (I) Axitinib, which inhibits the T315I mutation of BCR-ABL1, a main source of drug resistance, and (II) Asciminib, which has been developed as an allosteric BCR-ABL1 inhibitor, targeting an entirely different binding site, and as such does not compete for binding with other drugs. These drugs offer new treatment options. Methods: We measured the proliferation of KCL-22 cells exposed to imatinib–dasatinib, imatinib–asciminib and dasatinib–asciminib combinations and calculated combination index graphs for each case. Moreover, using the median–effect equation we calculated how much axitinib can reduce the growth advantage of T315I mutant clones in combination with available drugs. In addition, we calculated how much the total drug burden could be reduced by combinations using asciminib and other drugs, and evaluated which mutations such combinations might be sensitive to. Results: Asciminib had synergistic interactions with imatinib or dasatinib in KCL-22 cells at high degrees of inhibition. Interestingly, some antagonism between asciminib and the other drugs was present at lower degrees on inhibition. -

Second Generation Inhibitors of BCR- ABL for the Treatment of Imatinib- Resistant Chronic Myeloid Leukaemia
REVIEWS Second generation inhibitors of BCR- ABL for the treatment of imatinib- resistant chronic myeloid leukaemia Ellen Weisberg*, Paul W. Manley‡, Sandra W. Cowan-Jacob§, Andreas Hochhaus|| and James D. Griffin¶ Abstract | Imatinib, a small-molecule ABL kinase inhibitor, is a highly effective therapy for early-phase chronic myeloid leukaemia (CML), which has constitutively active ABL kinase activity owing to the expression of the BCR-ABL fusion protein. However, there is a high relapse rate among advanced- and blast-crisis-phase patients owing to the development of mutations in the ABL kinase domain that cause drug resistance. Several second-generation ABL kinase inhibitors have been or are being developed for the treatment of imatinib- resistant CML. Here, we describe the mechanism of action of imatinib in CML, the structural basis of imatinib resistance, and the potential of second-generation BCR-ABL inhibitors to circumvent resistance. The BCR-ABL oncogene, which is the product of the design of new drugs to circumvent resistance, and Philadelphia chromosome (Ph) 22q, encodes a chimeric several new agents have been developed specifically BCR-ABL protein that has constitutively activated ABL for this purpose. These compounds have been well tyrosine kinase activity; it is the underlying cause of characterized for efficacy against the mutant enzymes chronic myeloid leukaemia (CML)1–3. Whereas the 210 in preclinical studies, and impressive therapeutic activ- kDa BCR-ABL protein is expressed in patients with ity has now been reported for two second generation CML, a 190 kDa BCR-ABL protein, resulting from an drugs in phase I and II clinical trials in patients with *Dana Farber Cancer alternative breakpoint in the BCR gene, is expressed in imatinib-resistant CML. -

Characteristics of Trials and Regulatory Pathways Leading to US Approval Of
Health Policy 123 (2019) 721–727 Contents lists available at ScienceDirect Health Policy j ournal homepage: www.elsevier.com/locate/healthpol Characteristics of trials and regulatory pathways leading to US approval of innovative vs. non-innovative oncology drugs a,b,∗ b Kerstin Noëlle Vokinger , Aaron S. Kesselheim a Academic Chair for Public Law, Health Law, Digitalization and Health Policy, Faculty of Law, University of Zurich / Institute for Primary Care, University Hospital of Zurich and University of Zurich, Zurich, Switzerland b Program On Regulation, Therapeutics, and Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA a r a t i c l e i n f o b s t r a c t Article history: Background: Successful first-generation drugs can be converted with small alterations to second-¨ Received 17 December 2018 generation drugs,which¨ are cheaper to develop and may pose less financial risk for manufacturers due Received in revised form 22 April 2019 to already validated action mechanism and a well-defined consumer market. Accepted 4 June 2019 Methods: We found four classes of cancer drugs for first- and second generation products approved in the US: BCR-ABL tyrosine kinase inhibitors (TKI) for treatment of CML, ALK + TKI for NSCLC, CD20 monoclonal Keywords: antibodies for CLL, and HER2 monoclonal antibodies for breast cancer. We analyzed the characteristics Health policy of the clinical trials and the approval pathways for these 14 drugs. Public health Results: First-generation and 4 out of 5 s-generation BCR-ABL TKI drugs were granted expedited approval, First-generation drug while all drugs were approved based on single-arm trials. -

Bosutinib (Bosulif®)
bosutinib (Bosulif®) EOCCO POLICY Policy Type: PA/ SP Pharmacy Coverage Policy : EOCCO116 Description Bosutinib (Bosulif) is a tyrosine kinase inhibitor that inhibits the Bcr-Abl kinase which promotes chronic myelogenous leukemia (CML). It is also known to inhibit Src-family kinases including Src, Lyn, and Hck. Length of Authorization Initial: Three months Renewal: 12 months Quantity Limits Product Name Dosage Form Indication Quantity Limit 100 mg tablets CML, newly diagnosed 90 tablets/30 days 400 mg tablets chronic phase 30 tablets/30 days bosutinib (Bosulif) CML , resistant or intolerant 500 mg tablets 30 tablets/30 days to prior therapy Initial Evaluation I. Bosutinib (Bosulif) may be considered medically necessary when the following criteria below are met: A. Medication is prescribed by, or in consultation with, an oncologist or hematologist; AND B. Medication will not be used in combination with other oncologic medications (i.e., will be used as monotherapy); AND C. A diagnosis of chronic myelogenous leukemia (CML) when the following are met: 1. Newly diagnosed chronic phase Philadelphia chromosome-positive (Ph+) CML; OR 2. Chronic, accelerated, or blast phase Ph+ CML; AND i. Resistant or intolerant to prior treatment with a tyrosine kinase inhibitor [e.g. imatinib (Gleevec), dasatinib (Sprycel), nilotinib (Tasigna)] II. Bosutinib (Bosulif) is considered investigational when used for all other conditions, including but not limited to: A. Glioblastoma B. Dementia C. Non-small cell lung cancer D. Mesothelioma E. Bladder cancer F. Ovarian, peritoneal, uterine cervical cancer G. Thymoma H. Thymus cancer Renewal Evaluation I. Member has received a previous prior authorization approval for this agent through this health plan; AND II. -

Bosutinib (Bosulif) National Drug Monograph October 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives
Bosutinib Monograph Bosutinib (Bosulif) National Drug Monograph October 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism of Bosutinib is a dual SRC/ABL1 tyrosine kinase inhibitor with minimal inhibitory Action activity against KIT and platelet-derived growth factor (PDGFR). Indication(s) Under Review Bosutinib is a kinase inhibitor indicated for the treatment of adult patients with chronic, accelerated or blast phase Ph+ chronic myelogenous leukemia (CML) with resistance or intolerance to prior therapy. Dosage Form(s) Under Review 100 mg, 500 mg tablets REMS REMS No REMS Pregnancy Rating Category D Executive Summary Efficacy The primary endpoint of Major Cytogenetic Response (MCyR) at week 24 was reached by 31% of chronic phase CML patients that were imatinib-intolerant or imatinib-resistant. Two-year follow up of the chronic phase population resulted in 85% of patients achieving Complete Hematologic Response (CHR) while 59% achieved and/or maintained MCyR; at 2-yrs PFS 79%, with 2-yr OS 92%. Efficacy in Accelerated phase (AP) and Blast Phase (BP) CML is evidenced by the endpoint of CHR achieved by 30% of patients in AP and 15% of those in BP by week 48; Overall Hematologic Response (OHR) by week 48 was attained by 55% of AP and 28% of BP patients, respectively Safety Common adverse reactions include diarrhea, nausea, vomiting, thrombocytopenia, abdominal pain and rash. -

Recommendations of the 99Th SEC (Oncology & Haematology)
Recommendations of the 99th SEC (Oncology & Haematology) Meeting held on 21.07.2020 & 22.07.2020 at CDSCO, HQ New Delhi: Agenda File Name & Drug Firm Name Recommendations No Name, Strength New Drug Division The firm Presented their proposal along with the protocol for Phase IV study. After detailed deliberation, the committee recommended for grant of permission to conduct the proposed Phase IV study with the F. No. ND/MA/19/00009 following changes: M/s MSN Pvt. 1. Bosutinib Tablets 100 Ltd. 1. At least 50% of patients to be enrolled mg, 400 mg, 500 mg should be Imatinib resistant CML. 2. The drug should be provided free of cost to responders till progression even after completion of planned study as part of post trial access as per rules. 3. PI should be medical oncologist or Haematologist. Dr. Anusuya F. No 12-01/19-DC (Pt- Gehlot, Member Secretary, 322) The committee opined that the proposal needs 2. Institutional to be deliberated in SEC (Neurology) along Platelet Rich Plasma Ethics with experts from Blood Bank & ENT. (PRP) Committee, Dr. SNMC, Jodhpur In light of recommendation of SEC dated 29.05.2020, the firm presented the protocol for ND/MA/19/000010 M/s BDR multiple dose, steady state BE study. 3. Pharmaceuticals Rucaparib tablets Limited After detailed deliberation, the committee recommended for grant of permission to conduct the BE study as per protocol presented. SND Division The firm presented their proposal for additional indication along with request for local clinical trial waiver. The committee opined that there is an unmet SND/IMP/20/000015 medical need for this disease condition which is serious and life threatening. -
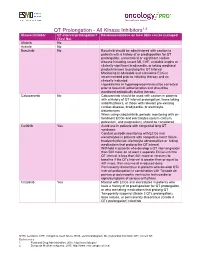
QT Prolongation of All Kinase Inhibitors
QT Prolongation - All Kinase Inhibitors1,2 Kinase Inhibitor QT-interval prolongation? Recommendations on how DDIs can be managed (Yes/ No) Afatinib No Axitinib No Bosutinib No Bosutinib should be administered with caution to patients with a history of or predisposition for QT prolongation, uncontrolled or significant cardiac disease including recent MI, CHF, unstable angina or clinically significant bradycardia, or taking medicinal products known to prolong the QT interval Monitoring is advisable and a baseline ECG is recommended prior to initiating therapy and as clinically indicated Hypokalemia or hypomagnesemia must be corrected prior to bosutinib administration and should be monitored periodically during therapy Cabozantinib No Cabozantinib should be used with caution in patients with a history of QT interval prolongation, those taking antiarrhythmics, or those with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances When using cabozantinib, periodic monitoring with on- treatment ECGs and electrolytes (serum calcium, potassium, and magnesium) should be considered Ceritinib Yes Avoid use in patients with congenital long QT syndrome Conduct periodic monitoring with ECGs and elecotrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities or taking medications that prolong the QT interval Withhold in patients who develop a QT interval greater than 500 msec on at least 2 separate ECGs until the QT interval is less than 481 msec or recovery to baseline if the QTc interval -

Repurposing of Anticancer Drugs: in Vitro and in Vivo Activities Against Schistosoma Mansoni Noemi Cowan1,2 and Jennifer Keiser1,2*
Cowan and Keiser Parasites & Vectors (2015) 8:417 DOI 10.1186/s13071-015-1023-y RESEARCH Open Access Repurposing of anticancer drugs: in vitro and in vivo activities against Schistosoma mansoni Noemi Cowan1,2 and Jennifer Keiser1,2* Abstract Background: Drug discovery for the neglected tropical disease schistosomiasis has a high priority. Anticancer drugs, especially protein kinase inhibitors, might serve as a starting point for drug discovery owing to the importance of protein kinases in helminth growth and development. Furthermore, the Schistosoma mansoni genome encodes several genes for targets of drugs marketed for human use, including several anticancer drugs. Methods: In this study, we screened the approved oncology drug set of the National Cancer Institute’s Developmental Therapeutic Program for antischistosomal activity. Drugs were tested in vitro against the larval and adult stage of S. mansoni.IC50 values and albumin binding were determined for active compounds. Lead compounds were tested in the chronic S. mansoni mouse model. Results: Eleven of the 114 compounds tested revealed IC50 values ≤ 10 μM against both S. mansoni stages. Five of these lost activity against adult S. mansoni in the presence of serum albumin. Of 6 compounds studied in vivo, the highest activity was observed from two kinase inhibitors trametinib, and vandetanib, which reduced worm burden by 63.6 and 48.1 % respectively, after a single oral dose of 400 mg/kg body weight. Conclusion: Our study has confirmed that oncology drugs possess antischistosomal activity. There is space for further investigation, including elucidation of the mechanisms of action of schistosome-active cancer drugs, application of different treatment courses, and structure-activity relationship studies for improving drug potency. -

Bosutinib for Pretreated Patients with Chronic Phase Chronic Myeloid Leukemia: Primary Results of the Phase 4 BYOND Study
Leukemia (2020) 34:2125–2137 https://doi.org/10.1038/s41375-020-0915-9 ARTICLE Chronic myelogenous leukemia Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: primary results of the phase 4 BYOND study 1 2 3 4 5 Andreas Hochhaus ● Carlo Gambacorti-Passerini ● Camille Abboud ● Bjørn Tore Gjertsen ● Tim H. Brümmendorf ● 6 1 7 8 9 B. Douglas Smith ● Thomas Ernst ● Pilar Giraldo-Castellano ● Ulla Olsson-Strömberg ● Susanne Saussele ● 10 11 12 13 12 Nathalie Bardy-Bouxin ● Andrea Viqueira ● Eric Leip ● T. Alexander Russell-Smith ● Jocelyn Leone ● 14 15 16 Gianantonio Rosti ● Justin Watts ● Francis J. Giles ● on behalf of the BYOND Study Investigators Received: 25 May 2020 / Revised: 1 June 2020 / Accepted: 4 June 2020 / Published online: 22 June 2020 © The Author(s) 2020. This article is published with open access Abstract Bosutinib is approved for newly diagnosed Philadelphia chromosome-positive (Ph+) chronic phase (CP) chronic myeloid leukemia (CML) and for Ph+ CP, accelerated (AP), or blast (BP) phase CML after prior treatment with tyrosine kinase inhibitors (TKIs). In the ongoing phase 4 BYOND study (NCT02228382), 163 CML patients resistant/intolerant to prior TKIs (n = 156 Ph+ CP CML, n = 4Ph+ AP CML, n = 3 Ph-negative/BCR-ABL1+ CML) received bosutinib 500 mg once ≥ + 1234567890();,: 1234567890();,: daily (starting dose). As of 1 year after last enrolled patient (median treatment duration 23.7 months), 56.4% of Ph CP CML patients remained on bosutinib. Primary endpoint of cumulative confirmed major cytogenetic response (MCyR) rate by 1 year was 75.8% in Ph+ CP CML patients after one or two prior TKIs and 62.2% after three prior TKIs. -

[Product Monograph Template
PRODUCT MONOGRAPH Pr BOSULIF® bosutinib tablets Tablets, 100 mg, 400 mg§ and 500 mg, Oral Protein-tyrosine kinase inhibitor § Not commercially available in Canada ® Wyeth LLC, Date of Revision: Pfizer Canada ULC, licensee 09 August 2019 17,300 Trans-Canada Highway Kirkland, Quebec H9J 2M5 ©Pfizer Canada ULC, 2019 Submission Control No: 227624 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION..........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................4 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS..................................................................................................15 DRUG INTERACTIONS ..................................................................................................30 DOSAGE AND ADMINISTRATION..............................................................................33 OVERDOSAGE ................................................................................................................36 ACTION AND CLINICAL PHARMACOLOGY ............................................................37 STORAGE AND STABILITY..........................................................................................40 -

Tyrosine Kinase Inhibitor (TKI) Flow Chart for the Treatment Of
Tyrosine Kinase Inhibitor (TKI) Therapeutic Flow Chart for the Treatment of CML A. Flow Chart •Imatinib 1sta •Dasatinib 2ndb •Nilotinib •Nilotinib 3rdb •Dasatinib •Bosutinib •Ponatinib B. Rationale and Supporting Evidence aFirst-line Therapy • Imatinib, the first approved TKI for CML, has been shown to provide durable responses in an 8-year follow up of the IRIS study with OS 93% [1] • Most long-term safety data of all agents (no new safety signals noted) • Dasatinib, a second-generation TKI, is supported by a 3-year follow up of DASISION with OS 93%; MMR 46% at 12 months [2] • Nilotinib, a second-generation TKI, is supported by a 3-year follow up of ENESTnd with OS 94%; MMR 44% at 12 months [3] • Impact of early response (MMR at 12 months) on survival remains to be determined • It is theorized that high-risk patients may benefit from a second-generation TKI as first-line therapy, but there is no supporting data and the optimal treatment for these patients remains to be determined • Unless a contraindication exists, imatinib should be the preferred first-line therapy bSecond-line and Subsequent Therapies • Guidelines (ELN, NCCN) recommend that choice of TKI take the following into consideration: side effect profile, kinase mutation profile, drug interactions, adherence issues, pre-existing comorbidities [4, 5, 6] • Second-line TKI recommended for patients on imatinib who do not meet treatment milestones at 12 months (BCR-ABL1 transcripts > 10% (IS), lack of at least partial cytogenetic response (PCyR) of bone marrow cytogenetics). • Evidence for use of bosutinib as a 4th TKI is supported by a small subset (n=3) of patients within a phase 2 trial (Khoury, 2012) and a retrospective evaluation (n=30) of patients in Spain (Garcia- Gutierrez, 2015) • Refer to Tyrosine Kinase Inhibitor Comparison Chart for CML for notable differences among agents October 2015 Page 1 C.