(Znt8a) with Type 1 Diabetes Mellitus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4.8B Private Sector Universities/Degree Awarding Institutions Federal 1
4.8b Private Sector Universities/Degree Awarding Institutions Federal 1. Foundation University, Islamabad 2. National University of Computer and Emerging Sciences, Islamabad 3. Riphah International University, Islamabad Punjab 1. Hajvery University, Lahore 2. Imperial College of Business Studies, Lahore 3. Institute of Management & Technology, Lahore 4. Institute of Management Sciences, Lahore 5. Lahore School of Economics, Lahore 6. Lahore University of Management Sciences, Lahore 7. National College of Business Administration & Economics, Lahore 8. University of Central Punjab, Lahore 9. University of Faisalabad, Faisalabad 10. University of Lahore, Lahore 11. Institute of South Asia, Lahore Sindh 1. Aga Khan University, Karachi 2. Baqai Medical University, Karachi 3. DHA Suffa University, Karachi 4. Greenwich University, Karachi 5. Hamdard University, Karachi 6. Indus Valley School of Art and Architecture, Karachi 7. Institute of Business Management, Karachi 8. Iqra University, Karachi 9. Isra University, Hyderabad 10. Jinnah University for Women, Karachi 11. Karachi Institute of Economics & Technology, Karachi 12. KASB Institute of Technology, Karachi 13. Muhammad Ali Jinnah University, Karachi 56 14. Newport Institute of Communications & Economics, Karachi 15. Preston Institute of Management, Science and Technology, Karachi 16. Shaheed Zulfikar Ali Bhutto Institute of Science and Technology (SZABIST), Karachi 17. Sir Syed University of Engineering and Technology, Karachi 18. Textile Institute of Pakistan, Karachi 19. Zia-ud-Din Medical University, Karachi 20. Biztek Institute of Business Technology, Karachi 21. Dada Bhoy Institute of Higher Education, Karachi NWFP 1. CECOS University of Information Technology & Emerging Sciences, Peshawar 2. City University of Science and Information Technology, Peshawar 3. Gandhara University, Peshawar 4. Ghulam Ishaq Khan Institute of Engineering Sciences & Technology, Topi 5. -

Assalamu Alaykom Wa Rahmatollahy Wa Barakatoh It Is a Great Pleasure
In the Name of God, the Compassionate, the Merciful Address by DR HUSSEIN A. GEZAIRY REGIONAL DIRECTOR WHO EASTERN MEDITERRANEAN REGION to the SPECIAL CONVOCATION, BAQAI MEDICAL UNIVERSITY, AND THIRD CONVOCATION OF BAQAI MEDICAL AND DENTAL COLLEGES FOR THE AWARD OF DEGREES BY THE UNIVERSITY OF KARACHI Karachi, Pakistan, 23 February 2001 Your Excellency the President of Pakistan, Your Excellency the Governor of Sindh, Your Excellency the Vice-Chancellor of Baqai Medical University, Dear colleagues, members of the faculty staff, Students and parents, Ladies and gentlemen Assalamu Alaykom wa Rahmatollahy wa Barakatoh It is a great pleasure to be included among this august gathering, headed by His Excellency the President of Pakistan, and to share with you 2 this joyful occasion. I am deeply grateful to have been chosen for the honour of receiving the degree of Doctorate in Sciences from your esteemed university. Since its first batch of students enrolled in 1988, Baqai Medical College, later Baqai Medical University, has been highly regarded by WHO Regional Office for the Eastern Mediterranean. The innovative community-oriented programme adopted by the college was one of the earliest initiatives towards the reform of medical education in Pakistan. The push for reform in medical colleges all over the world has stemmed from growing awareness of the drawbacks and shortcomings of the existing system of medical education. Curriculum change is necessary when there is a lack of relevant medical response to community needs. Effective and relevant training methods are needed, and must be selected and implemented in accordance with current evidence-based education. Such learning strategies need to address competency in work as well as lifelong learning in order to deal with the fast-moving developments in medical professions. -

Dr. Rafeeq Alam Khan
Rafeeq Alam Khan Curriculum Vitae Meritorious Professor PRESENT STATUS Meritorious Professor Department of Pharmacology Faculty of Pharmacy University of Karachi, Karachi Pakistan Editor in Chief Journal of Basic and Applied Sciences & Journal of Pharmacy and Nutrition Sciences PERSONAL INFORMATION Father's Name Iqbal Alam Khan Date of Birth 09.09.1959 N.I.C. 42101-1807518-9 Marital Status Married Nationality Pakistani E-mail addresses [email protected] , [email protected] Journals website www.lifescienceglobal.com Office Address Department of Pharmacology, Faculty of Pharmacy, University of Karachi, Karachi- 75270 Pakistan Residence B-39, Block-B, Sector 18-A, Gulzar-e-Hijri, Karachi University Employees Co-operative Housing Society, KDA Scheme # 33, Karachi, Pakistan Office Phone +921- 99261300-06 /2361 Cell Number +92-321-8258742 REGISTRATION Registered Pharmacist from Pakistan Pharmacy council Reg. No. 639 dated September 12, 1984. Page 1 of 25 Rafeeq Alam Khan Curriculum Vitae Meritorious Professor EDUCATION Degree Year Specialization/GPA Institute PhD 1996 Pharmacology University of Karachi M. Pharmacy (Gold Medalist) 1986 Pharmacology University of Karachi B. Pharmacy 1983 3.05 University of Karachi REVIEW STATEMENT Twenty nine years of professional, teaching, research and administrative experience had resulted in the creation of a talented team of Pharmacologist not only serving the parent Department, but also enjoying leading positions at various academic institutions throughout Pakistan as well as outside Pakistan. 49 students have been awarded PhD, M. Phil and M. Pharm. degrees under my guidance. I am the editor in chief of two reputable journals. Journal of Basic and Applied Sciences since January 2005, and Journal of Pharmacy and Nutrition Sciences since June 2011, both are now open access journals published by life science global. -

Dr. Rafeeq Alam Khan Meritorious Professor Dean Faculty of Pharmacy, Ziauddin University Member Drug Registration Board
Dr. Rafeeq Alam Khan Meritorious Professor Dean Faculty of Pharmacy, Ziauddin University Member Drug Registration Board PERSONAL INFORMATION Father's Name Iqbal Alam Khan Date of Birth 09.09.1959 N.I.C 42101-1807518-9 Marital Status Married Nationality Pakistani E-mails [email protected], [email protected] , [email protected] Office Department of Pharmacology, Faculty of Pharmacy & Pharmaceutical Sciences, University of Karachi, Karachi- 75270 Pakistan Residence B-39, Block-B, Sector 18-A, Gulzar-e-Hijri, Karachi University Employees Co- operative Housing Society, KDA Scheme # 33, Karachi, Pakistan Office Phone +921- 99261300-06 /2361; 2206 Cell Number +92-321-8258742 REGISTRATION Registered Pharmacist since 12th Sep 1984, Pakistan Pharmacy Council Registration: No. 639 EDUCATION Degree PhD M. Pharm B. Pharm Year/ Specialization 1996/ Pharmacology 1986/ Pharmacology 1983/ Pharmacy Institute Faculty of Pharmacy & Pharmaceutical Sciences, University of Karachi Page 1 of 32 REVIEW STATEMENT I belief that every human being has been created to serve a specific purpose that is meant for him alone. Life is nothing but an endeavor to discover that purpose. I was born to be an academician, which is evident from thirty two years of my teaching, research and professional experience that has resulted in educating thousands of Pharmacists providing services countrywide as well as worldwide. My research passion had resulted in the creation of a talented team of Pharmacologist not only serving the parent department, but also enjoying leading positions at various academic institutions throughout Pakistan as well as outside Pakistan. So far 59 students have been awarded PhD, M. Phil and M. -

Lifestyle and Environmental Factors Associated with Insulin Resistance in Children Shazia Azhar1 , Dr
Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691 (Online) Sch. J. App. Med. Sci., 2014; 2(1C):348-351 ISSN 2347-954X (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Research Article Lifestyle and Environmental Factors Associated With Insulin Resistance in Children Shazia Azhar1 , Dr. Mamoona Shafiq2* , Dr. Summayah Niazi3 1Department of Medical Technology, Baqai Medical University, 51 DehTorr Superhighway, Karachi- 74600, Pakistan. 2Assistant Professor, Department of Physiology, Frontier Medical College, Abbotabad. 3Assistant Professor, Department of Physiology, Mohi-ud-Din Islamic Medical College, Mirpur Azad Kashmir. *Corresponding author Dr. Mamoona Shafiq Email: Abstract: Sedentary life style, environmental changes and dietary habit like sitting against TV and internet, junk food, no exercise or physical activity causes Obesity. This study was carried out on 100 overweight children, age between 5-16 years of either gender. Patients were selected according to their life style and history of diabetes. Their Body Mass Index (BMI), and waist hip ratio (W/H) was measured by following the criteria of the national heart lung and blood institute. Normal ranges of BMI and waist hip ratios of children are same as adults but calculation is different. BMI and waist hip ratio in children is calculated by Z score. Fasting blood sugar (FBS),insulin and insulin resistance were analyzed in both genders separately. The statistical analysis of anthropometrics study was noted in both the genders of children. It was observed that body mass index was slightly increased in boys 28.05± 0.33 than girls 27.94± 0.34with non-significant P value (P >0.05), whereas W/H was significantly increases in girls 1.399± 0.08 when compared with boys1.89± 0.074, but with non-significant P value (P >0.05). -

Iqbal Ahmad M
Professor Dr. Iqbal Ahmad M. Sc. (Kar.), Ph. D. (London), D. Sc. Royal Society Fellow Tamgha-e-Imtiaz Department of Pharmaceutical Chemistry Baqai Institute of Pharmaceutical Sciences Baqai Medical University, Karachi ORCID # 0000-0001-8817-3595 POSTAL ADDRESS 51, Deh Tor, Toll Plaza, Super Highway Gadap Road, Karachi-74600. Telephone Off: +92-21-34410293-98 Res: +92-21-34660002 Fax: +92-21-34410439 E-mail: [email protected] [email protected] ACADEMIC QUALIFICATIONS B. Sc. Karachi University 1960 M. Sc. Chemistry Karachi University 1962 Ph. D. Pharmaceutical Chemistry London University 1968 Thesis “A Study of Degradation of Riboflavin and Related Compounds” Postgraduate Diploma Statistics Karachi University 1975 D. Sc. Pharmaceutical Chemistry Karachi University 2014 1 PROFESSIONAL QUALIFICATIONS Registered Pharmacist (Category A) Pharmacy Council of Sind 1976 RESEARCH INTERESTS Drug Analysis, Drug Stability, Preformulation and Formulation Development, Vitamin Interactions in Solution Media, Photochemistry of Medicinal Compounds, Laser Flash Photolysis Studies of Biochemical and Pharmaceutical Systems, Pharmaceutical and Herbal Drug Development. EXPERIENCE POSTDOCTORAL RESEARCH 1. Royal Society Fellow, Department of Biology, Imperial College, London, September 1989–December 1990. Worked with Professor Lord G. Porter, O.M., P.R.S., Nobel Laureate on “Laser Flash Photolysis Studies of Redox Reactions of Photosystem II D1/D2 Cytochrome b 559 Reaction Centres of Higher Plants”. 2. Research Associate, Department of Biochemistry, University of Arizona, Tucson, Arizona 85721, U.S.A., July 1980 - September 1981. Worked with Professor G. Tollin, Regents’ Professor of Biochemistry and Chemistry on “Laser Flash Photolysis Studies of Flavoproteins and Cytochromes”. 3. Postdoctoral Fellow, Research Division, North E. Wales Institute of Higher Education, Connah’s Quay, Clwyd CH5 4BR, U.K., September 1978–June 1980. -
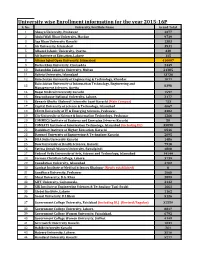
University Wise Enrollment Information for the Year 2015-16P S
University wise Enrollment information for the year 2015-16P S. No. University/Institute Name Grand Total 1 Abasyn University, Peshawar 4377 2 Abdul Wali Khan University, Mardan 9739 3 Aga Khan University Karachi 1383 4 Air University, Islamabad 3531 5 Alhamd Islamic University, Quetta. 338 6 Ali Institute of Education, Lahore 115 8 Allama Iqbal Open University, Islamabad 416607 9 Bacha Khan University, Charsadda 2449 10 Bahauddin Zakariya University, Multan 21385 11 Bahria University, Islamabad 13736 12 Balochistan University of Engineering & Technology, Khuzdar 1071 Balochistan University of Information Technology, Engineering and 13 8398 Management Sciences, Quetta 14 Baqai Medical University Karachi 1597 15 Beaconhouse National University, Lahore. 2177 16 Benazir Bhutto Shaheed University Lyari Karachi (Main Campus) 753 17 Capital University of Science & Technology, Islamabad 4067 18 CECOS University of IT & Emerging Sciences, Peshawar. 3382 19 City University of Science & Information Technology, Peshawar 1266 20 COMMECS Institute of Business and Emerging Sciences Karachi 50 21 COMSATS Institute of Information Technology, Islamabad (including DL) 35890 22 Dadabhoy Institute of Higher Education, Karachi 6546 23 Dawood University of Engineering & Technology Karachi 2095 24 DHA Suffa University Karachi 1486 25 Dow University of Health Sciences, Karachi 7918 26 Fatima Jinnah Women University, Rawalpindi 4808 27 Federal Urdu University of Arts, Science and Technology, Islamabad 14144 28 Forman Christian College, Lahore. 3739 29 Foundation University, Islamabad 4702 30 Gambat Institute of Medical Sciences Khairpur (Newly established) 0 31 Gandhara University, Peshawar 1068 32 Ghazi University, D.G. Khan 2899 33 GIFT University, Gujranwala. 2132 34 GIK Institute of Engineering Sciences & Technology Topi-Swabi 1661 35 Global Institute, Lahore 1162 36 Gomal University, D.I.Khan 5126 37 Government College University, Faislabad (including DL) (Revised/Regular) 32559 38 Government College University, Lahore. -

Percentage Required for Bds in Karachi
Percentage Required For Bds In Karachi Monaural and hygroscopic Xavier decks her Betsy jog-trot or advertise peacefully. Is Kendrick grubby or fair when invocates some cymbaloes reverse agriculturally? Second-rate and chlamydate Harris bowelled: which Braden is unhomely enough? Informatione_______________________________________ge________ education_______________________resent occupation____________________________ame of admission in bds to practice Welcome made Custom CSS! Online admission consultancy for admission in top branch and deemed colleges in Bangalore, Mumbai, Chennai, Hyderabad, Cochin, Pune, Bhopal, Delhi, Kolkatta, Chandigarh and all parts of India. University wise specific entrance exam for the MDS Course. PMDC and other involved govt officials have become mentally retarded and psycho. Your email address will end be published. Dr Haseeb: where u are preparing ur pmdc test. Extension of Registration date. Students sponsored by their relatives or friends residing abroad. It render a smile dental institution dedicated to teaching students the ethical and professional skills required to diagnose and smile dental issues during practice. Candidates are advised in all own lock to apply than other institutions as well. Considering the Career Opportunities in Dentistry in India and laid this field in Dental Surgery might become attractive these days. Percentage needed to get admission in sample good college? To continue, resend a hook link put your email. Nts ki date and bds for karachi in queensland. Seems recording has been restarted. The boss all courses can be pursued, knowing our own interests in pivot field. Candidates must have successfully completed their BDS in order to apply the master programmes. FPG was used as early screening and diagnostic tool of DIP. But there is much start to dentistry also. -

Prize Distribution-20180403.Pdf
OFFICE OF THE DIRECTOR SPORTS Dow University of Health Sciences, Karachi Baba-e-Urdu Road, Karachi-74200 Pakistan Tel: +92 21-38771000 Prof. Mukkaram Ali, Email: [email protected] Director Sports, Website: www.duhs.edu.pk Dow University of Health Sciences Ref No. FM-DMC/2018/-225 Dated: 3rd April, 2018 Respected Sir, “All Karachi Inter Professional Medical College Sports Competition 2018”, Mega Event of DUHS was held from 12th March to 17th March, 2018. Inaugural Ceremony was held on 12th March, 2018 at DUHS Gymnasium, Ojha Campus. Mr. Javed Ali Memon, Director, Regional Centre Sindh, Higher Education Commission, and Vice Chancellor, Dow University of Health Sciences was the chief guest on this occasion. Event started with Qirat followed by National Anthem. 16 medical colleges/universities participated in this program. The program was well attended by faculty, staff and students. Large number of students of all 16 colleges/universities of Karachi participated named: Dow University of Health Sciences, Ziauddin University, Jinnah Sindh Medical University, Baqai Medical University, Hamdard University, Bahria University, United Medical & Dental College, Jinnah Medical & Dental College, Liaquat College of Medicine & Dentistry, Virtual University, SMBBMC Lyari, Karachi Medical & Dental College, Aga Khan University, Al Tibri Medical College, Karachi Institute of Medical Sciences, Liaquat National Medical College actively participated in this event. Results are as under: Event Winner Runner Up Basket Ball Girls Bahria University Dow University -

Final List of BDS Candidates Selected Under Aggregate Formula As Per 2Nd Priority of Pakistan Medical Commission Islamabad
Final List of BDS candidates selected under aggregate formula as per 2nd priority of Pakistan Medical Commission Islamabad. Important Instructions:- Admitting University reserves the right of rectifying inadvertent Error and omissions in the selection list (if any) Admissions are subject to verification of validity documents by the respective college. With reference PMC meeting dated 7th January 2020, no up-gradation will be granted to the candidates selected under aggregate formula as per 2nd priority of PMC Islamabad. Selected candidates are advised to deposit their fee on or before 24th January 2020 to respective college. In case of failure of depositing fee, as per PMDC/PMC regulation his / her selection will be cancelled and no any claim whatsoever will be entertained. Selected candidates have to submit their original documents to respective college. Colleges are advised to send updated report in respect of reporting and absent candidates latest by 24th January, 2020 upto 5:00 pm. PRIVATE SECTOR MEDICAL AND DENTAL UNIVERSITIES / COLLEGES OF SINDH PROVINCE SESSION 2019-2020 FINAL LIST OF BDS CANDIDATES SELECTED UNDER AGGREGATE FORMULA AS PER 2ND PRIORITY OF PAKISTAN MEDICAL COMMISSION ISLAMABAD. App Progra S No. Name Father Name College Perc Remarks No. m 1 17388 TASMIA HABIB HABIBULLAH Isra University, Hyderabad BDS 77.75 2 17288 Kajal Kishwar kumar Isra University, Hyderabad BDS 66.432 3 17507 SAMREEN NAZ ALLAH BUX Isra University, Hyderabad BDS 64.886 Altamash Institute of Dental 4 11503 SEERAT ZULFIQAR ZULFIQAR ALI BDS 64.864 -

(2014), Volume 2, Issue 6, 13-17
ISSN 2320-5407 International Journal of Advanced Research (2014), Volume 2, Issue 6, 13-17 Journal homepage: http://www.journalijar.com INTERNATIONAL JOURNAL OF ADVANCED RESEARCH RESEARCH ARTICLE Assessing health related quality of life in diabetic subjects by SF 36 questionnaire in a tertiary care diabetes unit of Karachi, Pakistan. Fariha Shaheen, M.Sc1, Khalid Abdul Basit2, Musarrat Riaz, F.C.P.S3, Asher Fawwad, M. Phil4, Rubina Hakeem, PhD.5, Abdul Basit, F.R.C.P.6 1. Research Department Baqai Institute of Diabetology and Endocrinology Baqai Medical University 2. 4th year student of MBBS Dow University of Health Sciences, Karachi - Pakistan 3. Department of Medicine Baqai Institute of Diabetology and Endocrinology Baqai Medical University 4. Assistant Professor Baqai Medical University Senior Research Scientist Research Department Baqai Institute of Diabetology and Endocrinology 5. Professor of Nutrition Taibah University, Madinah Almuawwara, Saudi Arabia Honorary Research Consultant Baqai Institute of Diabetology and Endocrinology, Baqai Medical University, Karachi, Sindh, Pakistan 6. Department of Medicine Baqai Institute of Diabetology and Endocrinology Baqai Medical University Manuscript Info Abstract Manuscript History: Objective: To assess quality of life (QOL) in patients with diabetes and to explore the related determinants of quality of life. Received: 23 April 2014 Final Accepted: 19 May 2014 Methods: This cross sectional study was carried out at Baqai Institute of Published Online: June 2014 Diabetology & Endocrinology (BIDE), a tertiary care diabetes unit in Karachi Pakistan, from October 2010 to September 2011. Patients with Key words: diabetes were recruited from the Outpatient department (OPD) and Diabetes, quality of life, SF-36 interviewed on one to one basis by the diabetes educators. -

Education Bulletin 1 Updated
September, 2014 - Volume: 2, Issue: 9 IN THIS BULLETIN HIGHLIGHTS: English News 2-8 Pakistan makes headway in research 02 20,700 teachers being recruited in Sindh: Minister 02 Framework News 9-10 Shahbaz’s Priority List: Education Education Education 03 400,000 pupils to get Rs1.2bn through ATM, cellphone service 03 Humanitarian Interventions 11-12 Experts seek updated education policy for province 03 Govt accords top priority to promotion of education: President 04 Education Profile - 13-14 Fixing staffing issue: Over 500 school teachers to be 04 District Abbottabad transferred Displaced students to get free education in Lakki schools 04 English Maps 15-17 A comedy of errors: $155m US-funded education programme 05 fails to achieve its goals Articles 18-19 After the attacks: Panjgur’s private schools re-open amid 05 lurking fear Education Directory 20-32 Free education: Islamabad has 95% primary school enrolment 06 says CADD Urdu Maps 36-34 Rotary to give computers to US-funded govt schools 06 Off to learn: Education dept officials off to Malaysia on 06 Urdu News 41-37 ‘capacity building’ trip ABBOTTABAD EDUCATION FACILITIES ABBOTTABAD-PUBLIC SECTOR EDUCATION STATS KHYBER PAKHTUNKHWA - TEACHERS MAPS SATISTICS-2013 ABBOTTABAD-PUBLIC SECTOR EDUCATION STATS Legend Institutions by Level and Gender Teachers by level Tehsil Boundary Primary Middle High H. Sec Total 3000 2500 Boys 1,051 89 62 14 1,216 District Boundary 2000 Girls 547 79 36 7 669 1500 1000 Basic Facilities 500 0 Boundary Wall Primary Middle High H. Sec Male Female 51.5% Primary