Agricultural Biotechnology: Overview, Regulation, and Selected Policy Issues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
U.S. V. Bayer AG and Monsanto Company Comment: the Sierra Club
ATTN: Kathleen S. O'Neill Chief, Transportation, Energy & Agriculture Section Antitrust Division United States Department of Justice 450 5th Street, NW, Suite 800 Washington, DC 20530 Petition in opposition to proposed U.S. v. Bayer AG and Monsanto Company settlement and merger: A merger of agrochemical giants Bayer and Monsanto would create the world's largest seed and pesticide maker. I am afraid this move will reduce competition, raise prices for consumers and farmers, and result in an unacceptable degree of control over the agricultural industry and our food supply. I am very concerned about pollinators and the increased risks to bees, butterflies and birds with the increase of Bayer's neonicotinoids. Both companies produce corn products engineered to imply the use of harmful pesticides they manufacture. The production of corn uses high amounts of nitrogen- based fertilizers and the excess sediment is contaminating our waterways, therefore I am deeply worried about increased corn production from this merger. The heavy nutrient runoff from corn is widely attributed to exacerbating the marine "Dead Zone" in the Gulf of Mexico, in which algal blooms create hypoxic conditions wherein oxygen concentration is in such low levels that marine life suffocates and dies. I urge the Department of Justice to do more prevent the Bayer-Monsanto seed and pesticide platform from growing too strong by stopping this merger. If this merger is allowed, it should require more pesticide and seed divestments in order to protect our agriculture and food supply. This merger is anti-competition, if it is approved it will fail to protect farmers, consumers and the environment by allowing further consolidation of the industrial agriculture sector. -

Welfare Issues with Genetic Engineering and Cloning of Farm Animals
WellBeing International WBI Studies Repository 2006 Welfare Issues with Genetic Engineering and Cloning of Farm Animals The Humane Society of the United States Follow this and additional works at: https://www.wellbeingintlstudiesrepository.org/ hsus_reps_impacts_on_animals Part of the Animal Studies Commons, Bioethics and Medical Ethics Commons, and the Other Genetics and Genomics Commons Recommended Citation The Humane Society of the United States, "Welfare Issues with Genetic Engineering and Cloning of Farm Animals" (2006). IMPACTS ON FARM ANIMALS. 21. https://www.wellbeingintlstudiesrepository.org/hsus_reps_impacts_on_animals/21 This material is brought to you for free and open access by WellBeing International. It has been accepted for inclusion by an authorized administrator of the WBI Studies Repository. For more information, please contact [email protected]. Farm Animal Welfare • The HSUS 2100 L St., N.W. • Washington, DC 20037 • HSUS.org 202-452-1100 • F: 301-258-3081 • FarmAnimalWelfare.org An HSUS Report: Welfare Issues with Genetic Engineering and Cloning of Farm Animals Abstract Developments in biotechnology have raised new concerns about animal welfare, as farm animals now have their genomes modified (genetically engineered) or copied (cloned) to propagate certain traits useful to agribusiness, such as meat yield or feed conversion. These animals suffer from unusually high rates of birth defects, disabili- ties, and premature death. Genetically engineering farm animals for greater bone strength or reduced pain reception, for example, may result in improved well-being, yet the broad use of this technology often causes increased suffering. The limited success of gene insertion techniques can result in genes failing to reach target cells and finishing in cells of unintended organs, and abnormally developed embryos may die in utero, be infertile, or born with development defects, attributable in part to insertional problems. -

Cloning: Adult Vs. Embryonic Cells and Techniques Employed
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Supervised Undergraduate Student Research Chancellor’s Honors Program Projects and Creative Work Spring 5-2001 Cloning: Adult vs. Embryonic Cells and Techniques Employed Rebecca Ruth Frazor University of Tennessee-Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_chanhonoproj Recommended Citation Frazor, Rebecca Ruth, "Cloning: Adult vs. Embryonic Cells and Techniques Employed" (2001). Chancellor’s Honors Program Projects. https://trace.tennessee.edu/utk_chanhonoproj/459 This is brought to you for free and open access by the Supervised Undergraduate Student Research and Creative Work at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Chancellor’s Honors Program Projects by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. UNIVERSITY HONORS PROGRAM SENIOR PROJECT - APPROVAL Name: Getec eCA f?- f(CAZOr College: AQn eMi:hA rv Department: A1 (met I SCi!A1w -J Faculty Mentor: F. N-S ChV\C ¥: PROJECT TITLE: C10l/1\(}q : Adult \[) ' fmmrVVl l0 C l, (( ~ ~rd the. ~ Qr\L\A<; Icchn \ quc ~ Emplo\( f d I have reviewed this completed senior honors thesis with this student and certify that it is a project commens rate with rs level undergraduate research in this field. Signed: ;. ./-1 Faculty Mentor Date: _-+-'""-+----L-___ Comments (Optional): Cloning: Adult vs. Embryonic Cells and Various Techniques Employed Rebecca R. Frazor Mentor: F. Neal Schrick Senior Honors Project May 2001 Abstract The cloning of amphibians and mainly mammals is discussed focusing on somatic cell usage. Significant progress has been made in recent years and this is outlined with an emphasis on techniques and their differences. -

Recombinant DNA and Elements Utilizing Recombinant DNA Such As Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology
Fact Sheet Describing Recombinant DNA and Elements Utilizing Recombinant DNA Such as Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology Compiled and/or written by Amy B. Vento and David R. Gillum Office of Environmental Health and Safety University of New Hampshire June 3, 2002 Introduction Recombinant DNA (rDNA) has various definitions, ranging from very simple to strangely complex. The following are three examples of how recombinant DNA is defined: 1. A DNA molecule containing DNA originating from two or more sources. 2. DNA that has been artificially created. It is DNA from two or more sources that is incorporated into a single recombinant molecule. 3. According to the NIH guidelines, recombinant DNA are molecules constructed outside of living cells by joining natural or synthetic DNA segments to DNA molecules that can replicate in a living cell, or molecules that result from their replication. Description of rDNA Recombinant DNA, also known as in vitro recombination, is a technique involved in creating and purifying desired genes. Molecular cloning (i.e. gene cloning) involves creating recombinant DNA and introducing it into a host cell to be replicated. One of the basic strategies of molecular cloning is to move desired genes from a large, complex genome to a small, simple one. The process of in vitro recombination makes it possible to cut different strands of DNA, in vitro (outside the cell), with a restriction enzyme and join the DNA molecules together via complementary base pairing. Techniques Some of the molecular biology techniques utilized during recombinant DNA include: 1. -

Direct Cloning and Refactoring of a Silent Lipopeptide Biosynthetic Gene Cluster Yields the Antibiotic Taromycin A
Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A Kazuya Yamanakaa,b, Kirk A. Reynoldsa,c, Roland D. Kerstena, Katherine S. Ryana,d, David J. Gonzaleze, Victor Nizete,f, Pieter C. Dorresteina,c,f, and Bradley S. Moorea,f,1 aScripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92093; bYokohama Research Center, JNC Corporation, Yokohama 236-8605, Japan; cDepartment of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA 92093; dDepartment of Chemistry, University of British Columbia, Vancouver, BC, Canada V62 1Z4; and Departments of ePediatrics and fSkaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, CA 92093 Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved December 23, 2013 (received for review October 23, 2013) Recent developments in next-generation sequencing technologies genes (9, 10). Synthetic biology approaches also can be used to have brought recognition of microbial genomes as a rich resource refactor orphan gene clusters by optimizing promoters, tran- for novel natural product discovery. However, owing to the scar- scriptional regulation, ribosome-binding sites, and even codon city of efficient procedures to connect genes to molecules, only use (11). Examples of successful regulatory gene manipulation to a small fraction of secondary metabolomes have been investigated elicit the production of new natural products from silent bio- to date. Transformation-associated recombination (TAR) cloning synthetic gene clusters in WT strains have been reported in takes advantage of the natural in vivo homologous recombination bacteria and fungi (12, 13); however, this approach requires the of Saccharomyces cerevisiae to directly capture large genomic loci. -

Dolly the Sheep – the First Cloned Adult Animal
DOLLY THE SHEEP – THE FIRST CLONED ADULT ANIMAL NEW TECHNOLOGY FOR IMPROVING LIVESTOCK From Squidonius via Wikimedia Commons In 1996, University of Edinburgh scientists celebrated the birth of Dolly the Sheep, the first mammal to be cloned using SCNT cloning is the only technology adult somatic cells. The Edinburgh team’s success followed available that enables generation of 99.8% its improvements to the single cell nuclear transfer (SCNT) genetically identical offspring from selected technique used in the cloning process. individuals of adult animals (including sterilized animals). As such, it is being Dolly became a scientific icon recognised worldwide and exploited as an efficient multiplication tool SCNT technology has spread around the world and has been to support specific breeding strategies of used to clone multiple farm animals. farm animals with exceptionally high genetic The cloning of livestock enables growing large quantities of value. the most productive, disease resistant animals, thus providing more food and other animal products. Sir Ian Wilmut (Inaugural Director of MRC Centre for Regeneration and Professor at CMVM, UoE) and colleagues worked on methods to create genetically improved livestock by manipulation of stem cells using nuclear transfer. Their research optimised interactions between the donor nucleus and the recipient cytoplasm at the time of fusion and during the first cell cycle. Nuclear donor cells were held in mitosis before being released and used as they were expected to be passing through G1 phase. CLONING IN COMMERCE, CONSERVATION OF AGRICULTURE AND PRESERVATION ANIMAL BREEDS OF LIVESTOCK DIVERSITY Cloning has been used to conserve several animal breeds in the recent past. -

The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-Dupont, and Chemchina-Syngenta
Research Brief October 2018 The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-DuPont, and ChemChina-Syngenta DISRUPT ECOSYSTEM ACCLERATE MONOPOLY THE EFFECTS OF CORPORATE CONSOLIDATION UNDERMINE FOOD SECURITY HARM SMALL PRODUCERS HAASINSTITUTE.BERKELEY.EDU This publication is published by the Haas Institute for a Fair and Inclusive Society at UC Berkeley This research brief is part of the Haas Institute's Shahidi Project from the Global Justice Program. The Shahidi Project (Shahidi is a Swahili word meaning “witness”) intends to demystify the power structures and capacities of transnational food and agricultural corporations within our food system. To that end, researchers have developed a robust database focusing on ten of the largest food and agricultural corporations in the world. See more at haasinstitute.berkeley.edu/shahidi. About the Authors Copyeditor Support Elsadig Elsheikh is the director Marc Abizeid Special thanks to the Food of the Global Justice program and Farm Communications at the Haas Institute for a Infographics Fund, which provided the seed Fair and Inclusive Society at Samir Gambhir funding for the Shahidi project. the University of California- Berkeley, where he oversees Report Citation Contact the program’s projects and Elsadig Elsheikh and Hossein 460 Stephens Hall research on corporate power, Ayazi. “The Era of Corporate Berkeley, CA 94720-2330 food system, forced migration, Consolidation and The End of Tel 510-642-3326 human rights, Islamophobia, Competition: Bayer-Monsanto, haasinstitute.berkeley.edu structural marginality and Dow-DuPont, and ChemChina- inclusion, and trade and Syngenta.” Haas Institute for development. a Fair and Inclusive Society at the University of California, Hossein Ayazi, PhD, is a Berkeley, CA. -

Genetic Engineering and Sustainable Crop Disease Management: Opportunities for Case-By-Case Decision-Making
sustainability Review Genetic Engineering and Sustainable Crop Disease Management: Opportunities for Case-by-Case Decision-Making Paul Vincelli Department of Plant Pathology, 207 Plant Science Building, College of Agriculture, Food and Environment, University of Kentucky, Lexington, KY 40546, USA; [email protected] Academic Editor: Sean Clark Received: 22 March 2016; Accepted: 13 May 2016; Published: 20 May 2016 Abstract: Genetic engineering (GE) offers an expanding array of strategies for enhancing disease resistance of crop plants in sustainable ways, including the potential for reduced pesticide usage. Certain GE applications involve transgenesis, in some cases creating a metabolic pathway novel to the GE crop. In other cases, only cisgenessis is employed. In yet other cases, engineered genetic changes can be so minimal as to be indistinguishable from natural mutations. Thus, GE crops vary substantially and should be evaluated for risks, benefits, and social considerations on a case-by-case basis. Deployment of GE traits should be with an eye towards long-term sustainability; several options are discussed. Selected risks and concerns of GE are also considered, along with genome editing, a technology that greatly expands the capacity of molecular biologists to make more precise and targeted genetic edits. While GE is merely a suite of tools to supplement other breeding techniques, if wisely used, certain GE tools and applications can contribute to sustainability goals. Keywords: biotechnology; GMO (genetically modified organism) 1. Introduction and Background Disease management practices can contribute to sustainability by protecting crop yields, maintaining and improving profitability for crop producers, reducing losses along the distribution chain, and reducing the negative environmental impacts of diseases and their management. -

A Short History of DNA Technology 1865 - Gregor Mendel the Father of Genetics
A Short History of DNA Technology 1865 - Gregor Mendel The Father of Genetics The Augustinian monastery in old Brno, Moravia 1865 - Gregor Mendel • Law of Segregation • Law of Independent Assortment • Law of Dominance 1865 1915 - T.H. Morgan Genetics of Drosophila • Short generation time • Easy to maintain • Only 4 pairs of chromosomes 1865 1915 - T.H. Morgan •Genes located on chromosomes •Sex-linked inheritance wild type mutant •Gene linkage 0 •Recombination long aristae short aristae •Genetic mapping gray black body 48.5 body (cross-over maps) 57.5 red eyes cinnabar eyes 67.0 normal wings vestigial wings 104.5 red eyes brown eyes 1865 1928 - Frederick Griffith “Rough” colonies “Smooth” colonies Transformation of Streptococcus pneumoniae Living Living Heat killed Heat killed S cells mixed S cells R cells S cells with living R cells capsule Living S cells in blood Bacterial sample from dead mouse Strain Injection Results 1865 Beadle & Tatum - 1941 One Gene - One Enzyme Hypothesis Neurospora crassa Ascus Ascospores placed X-rays Fruiting on complete body medium All grow Minimal + amino acids No growth Minimal Minimal + vitamins in mutants Fragments placed on minimal medium Minimal plus: Mutant deficient in enzyme that synthesizes arginine Cys Glu Arg Lys His 1865 Beadle & Tatum - 1941 Gene A Gene B Gene C Minimal Medium + Citruline + Arginine + Ornithine Wild type PrecursorEnz A OrnithineEnz B CitrulineEnz C Arginine Metabolic block Class I Precursor OrnithineEnz B CitrulineEnz C Arginine Mutants Class II Mutants PrecursorEnz A Ornithine -
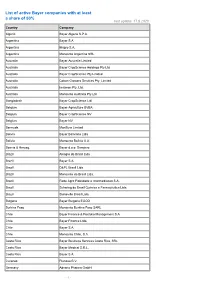
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

How They Cloned a Sheep
How They Cloned A Sheep How They Cloned A Sheep This text is provided courtesy of the American Museum of Natural History. How they cloned a sheep 1. Scientists took udder cells from Dolly's DNA mother. They let the cells multiply and then they stopped the process when they had divided enough. 2. They took an egg cell from a different sheep and removed the nucleus. 3. They put one udder cell next to the egg cell without a nucleus and joined them using electricity. The egg cell now contained all the udder cell's DNA. 4.The egg cell divided until it developed into an embryo. An embryo is the early stage of an animal before it has been born or hatched. This embryo was placed inside a third sheep. Five months later, this sheep gave birth to Dolly. ReadWorks.org · © 2015 ReadWorks®, Inc. All rights reserved. How They Cloned A Sheep - Comprehension Questions Answer Key 1. What did scientists remove from the egg cell of a sheep? A. the nucleus B. the embryo C. DNA D. udder cells 2. This passage describes the sequence of events involved in cloning a sheep. What happened after the scientists took udder cells and an egg cell from different sheep? A. The egg cell was joined to an udder cell using electricity. B. The embryo was placed inside another sheep. C. Scientists took udder cells from Dolly's DNA mother. D. The egg cell divided until it formed an embryo. 3. As part of the process of cloning a sheep, scientists placed the embryo inside a sheep, which eventually gave birth to Dolly. -

Barriers to Adoption of GM Crops
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2021 Barriers to Adoption of GM Crops Madeline Esquivel Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Agricultural Education Commons Recommended Citation Esquivel, Madeline, "Barriers to Adoption of GM Crops" (2021). Creative Components. 731. https://lib.dr.iastate.edu/creativecomponents/731 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Barriers to Adoption of GM Crops By Madeline M. Esquivel A Creative Component submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Plant Breeding Program of Study Committee: Walter Suza, Major Professor Thomas Lübberstedt Iowa State University Ames, Iowa 2021 1 Contents 1. Introduction ................................................................................................................................. 3 2. What is a Genetically Modified Organism?................................................................................ 9 2.1 The Development of Modern Varieties and Genetically Modified Crops .......................... 10 2.2 GM vs Traditional Breeding: How Are GM Crops Produced?