Antimicrobial and Cytotoxic Activities of Hopea Utilis Fruits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government of India Ministry of Housing & Urban Affairs
GOVERNMENT OF INDIA MINISTRY OF HOUSING & URBAN AFFAIRS LOK SABHA UNSTARRED QUESTION No. 2503 TO BE ANSWERED ON JANUARY 2, 2018 URBAN INFRASTRUCTURE PROJECTS No. 2503. SHRI R. GOPALAKRISHNAN: Will the Minister of HOUSING & URBAN AFFAIRS be pleased to state: (a) whether the Government has granted approval and released funds for implementing a number of urban infrastructure projects of Tamil Nadu; (b) if so, the details thereof along with the funds allocated/released for the said purpose during the last three years and the current year, city-wise including Madurai city in Tamil Nadu; and (c) the present status of those projects and the steps taken/being taken for expediting these projects? ANSWER THE MINISTER OF STATE (INDEPENDENT CHARGE) IN THE MINISTRY OF HOUSING & URBAN AFFAIRS (SHRI HARDEEP SINGH PURI) (a) to (c) Yes Madam. The Ministry of Housing & Urban Affairs has approved and released funds for implementing urban infrastructure projects in Tamil Nadu under its various schemes, viz., Atal Mission for Rejuvenation and Urban Transformation (AMRUT), Smart Cities Mission (SCM), Page 1 of 2 Heritage City Development and Augmentation Yojana (HRIDAY), Swacchh Bharat Mission – Urban [SBM (U)], Urban Infrastructure Development in Satellite Towns around Seven Mega Cities (UIDSST), Urban Transport (UT), Pradhan Mantri Awas Yojana-Urban [PMAY (U)] and Jawaharlal Nehru National Urban Renewal Mission (JnNURM). Under AMRUT, the Ministry of Housing & Urban Affairs does not approve projects for individual cities but accords approval to the State Annual Action Plans (SAAPs) only. Selection, approval and implementation of individual projects is done by State Government. Further, the Ministry of Housing & Urban Affairs does not release central share of funds city-wise, but funds are released State-wise. -

Biology ABSTRACT Distribution of Pteropodid Bats in Tirunelveli
Research Paper Volume : 2 | Issue : 3 | Mar 2013Biology • ISSN No 2277 - 8179 Distribution of Pteropodid Bats in KEYWORDS: Distribution, Richness, Tirunelveli, Tuticorin and Kanyakumari Megachiroptera, C. sphinx, P.giganteus Districts of Tamilnadu, South India Sudhakaran, M. R Department of Zoology, Sri Paramakalyani College, Alwarkurichi-627 412, Tamilnadu, India Paramanantha D., School of Biological Sciences, Madurai Kamaraj University, Madurai 625 012, Tamilnadu, Swami Doss India. Parvathiraj, P Department of Zoology, Sri Paramakalyani College, Alwarkurichi-627 412, Tamilnadu, India. ABSTRACT This study was mainly done to access the distributional pattern of megachiropterans in the plains of Tirunelveli, Tuticorin and Kanyakumari districts of Tamilnadu, South India. Three species of megachiropterans ie., C. sphinx, R. leschenaulti and P. giganteus was observed to present in this area. On evaluating the species richness, Tirunelveli district was observed to have a higher value (C. sphinx Dmg = 1.497 , R. leschenaulti Dmg = 0.724 and P. giganteus Dmg = 0.609) than that of the other two districts. INTRODUCTION of Tirunelveli, Kanyakumari and Ramanathapuram. This district Bats form the second largest mammalian order, representing a has also got diverse geographical and physical features such as quarter of all mammals [1]. They belong to the Order Chirop- lofty mountains and low plains, dry Teri structures, seacoast tera and on the basis of their specialization in feeding habits and sub orders Megachiroptera and Microchiroptera. Megachirop- and thorny scrub jungles. It lies in 08º 45’of N latitude and 78º teramorphological are predominantly adaptations; fruit bats eaters are and broadly Microchiroptera, classified into which two 3.13’ Kanyakumari E longitude. district form the majority of bat species globally, feed mostly on insects. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

AND IT's CONTACT NUMBER Tirunelveli 0462
IMPORTANT PHONE NUMBERS AND EMERGENCY OPERATION CENTRE (EOC) AND IT’S CONTACT NUMBER Name of the Office Office No. Collector’s Office Control Room 0462-2501032-35 Collector’s Office Toll Free No. 1077 Revenue Divisional Officer, Tirunelveli 0462-2501333 Revenue Divisional Officer, Cheranmahadevi 04634-260124 Tirunelveli 0462 – 2333169 Manur 0462 - 2485100 Palayamkottai 0462 - 2500086 Cheranmahadevi 04634 - 260007 Ambasamudram 04634- 250348 Nanguneri 04635 - 250123 Radhapuram 04637 – 254122 Tisaiyanvilai 04637 – 271001 1 TALUK TAHSILDAR Taluk Tahsildars Office No. Residence No. Mobile No. Tirunelveli 0462-2333169 9047623095 9445000671 Manoor 0462-2485100 - 9865667952 Palayamkottai 0462-2500086 - 9445000669 Ambasamudram 04634-250348 04634-250313 9445000672 Cheranmahadevi 04634-260007 - 9486428089 Nanguneri 04635-250123 04635-250132 9445000673 Radhapuram 04637-254122 04637-254140 9445000674 Tisaiyanvilai 04637-271001 - 9442949407 2 jpUney;Ntyp khtl;lj;jpy; cs;s midj;J tl;lhl;rpah; mYtyfq;fspd; rpwg;G nray;ghl;L ikaq;fspd; njhiyNgrp vz;fs; kw;Wk; ,izatop njhiyNgrp vz;fs; tpguk; fPo;f;fz;lthW ngwg;gl;Ls;sJ. Sl. Mobile No. with Details Land Line No No. Whatsapp facility 0462 - 2501070 6374001902 District EOC 0462 – 2501012 6374013254 Toll Free No 1077 1 Tirunelveli 0462 – 2333169 9944871001 2 Manur 0462 - 2485100 9442329061 3 Palayamkottai 0462 - 2501469 6381527044 4 Cheranmahadevi 04634 - 260007 9597840056 5 Ambasamudram 04634- 250348 9442907935 6 Nanguneri 04635 - 250123 8248774300 7 Radhapuram 04637 – 254122 9444042534 8 Tisaiyanvilai 04637 – 271001 9940226725 3 K¡»a Jiw¤ jiyt®fë‹ bršngh‹ v§fŸ mYtyf vz; 1. kht£l M£Á¤ jiyt®, ÂUbešntè 9444185000 2. kht£l tUthŒ mYty®, ÂUbešntè 9445000928 3. khefu fhtš Miza®, ÂUbešntè 9498199499 0462-2970160 4. kht£l fhtš f§fhâ¥ghs®, ÂUbešntè 9445914411 0462-2568025 5. -

Dr.AG Murugesan
Dr.A.G. Murugesan PhD FNABS FAZ FAEB FASc AW FST FSESc TANSA Professor and Head i) Research Interests Environmental Pollution and Mitigation, Bioremediation, Water Resources Management, Environmental Impact Assessment, Biomonitoring of river pollution, Use and throw plastic Eradication, Bioenergy, Biopolymer (PHB) and Ethanol Production from Industrial Wastes and Aquatic weeds, Hydrogen generation from Agrowastes, Solid Waste Management, Biocontrol of Water hyacinth, studies on flora and fauna in Industrial areas. ii) Government Awards National award for Innovation in Polymeric Material from Natural Resource by Ministry of Chemicals & Petrochemicals, Govt. of India (2015-16) TANSA Award - 2004 for Environment – by Higher Education Department, Government of Tamil Nadu. Environmental Protection Award - 2003 - 2004 – by Department of Environment, Government of Tamil Nadu. Academic Excellence Award – 2008 by M. S. University iii) Prestigious Other Awards Shri P.K.Das Best Faculty and Life Time Achievement Award – 2011 by Nehru Group of Institutions Rotary – Green Ambassador Award 2012 iv) Other Awards Best Environmental Scientist Award by Rotary International. Distinguished Environmental Scientist Award – TNUN Trust, Chennai Mahakavi Bharathiar Best Teacher Award by the Bharathiar International Service Trust. NMS Centenary Science Achievement Award Best Toxicologist Award by Federation of South Indian Traditional Medical Practitioners. Alumni Achiever Award (2004) - SPK College. Vocational Excellence Award (Rotary International) - 2005. Best Alumini Achiever Award (2005) - St. Xavier’s College. Best Research Paper Presentation Award (TNSC). Alumini Achiever Award (2015) - SPK College. CONFERENCES / WORKSHOPS – Organized Workshop on Biological Control of Water Hyacinth, Tirunelveli- May 2002 Workshop on Traditional Medicine, Tirunelveli- Aug. 2002. Seminar on Conservation of Water Resources, Thirumalayappapuram- Dec. 2002. Workshop on Production of Organic manure, Alwarkurichi- Feb. -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2015 [Price: Rs. 280.80 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 18] CHENNAI, SUNDAY, AUGUST 16, 2015 Aadi 31, Manmadha, Thiruvalluvar Aandu-2046 CONTENTS DEPARTMENTAL TESTS—RESULTS, MAY 2015 Name of the Tests and Code Numbers Pages. Pages. Second Class Language Test (Full Test) Part ‘A’ The Tamil Nadu Wakf Board Department Test First Written Examination and Viva Voce Parts ‘B’ ‘C’ Paper Detailed Application (With Books) (Test 2425-2434 and ‘D’ (Test Code No. 001) .. .. .. Code No. 113) .. .. .. .. 2661 Second Class Language Test Part ‘D’ only Viva Departmental Test in the Manual of the Firemanship Voce (Test Code No. 209) .. .. .. 2434-2435 for Officers of the Tamil Nadu Fire Service First Paper & Second Paper (Without Books) Third Class Language Test - Hindi (Viva Voce) (Test Code No. 008 & 021) .. .. .. (Test Code 210), Kannada (Viva Voce) 2661 (Test Code 211), Malayalam (Viva Voce) (Test The Agricultural Department Test for Members of Code 212), Tamil (Viva Voce) (Test Code 213), the Tamil Nadu Ministerial Service in the Telegu (Viva Voce) (Test Code 214), Urdu (Viva Agriculture Department (With Books) Test Voce) (Test Code 215) .. .. .. 2435-2436 Code No. 197) .. .. .. .. 2662-2664 The Account Test for Subordinate Officers - Panchayat Development Account Test (With Part-I (With Books) (Test Code No. 176) .. 2437-2592 Books) (Test Code No. 202).. .. .. 2664-2673 The Account Test for Subordinate Officers The Agricultural Department Test for the Technical Part II (With Books) (Test Code No. 190) .. 2593-2626 Officers of the Agriculture Department Departmental Test for Rural Welfare Officer (With Books) (Test Code No. -
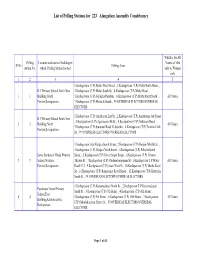
List of Polling Stations for 223 Alangulam Assembly Constituency
List of Polling Stations for 223 Alangulam Assembly Constituency Whether for All Polling Location and name of building in Voters or Men Sl.No Polling Areas station No. which Polling Station located only or Women only 1 2 3 4 5 1.Keelapavoor (T.P) Melur West Street. , 2.Keelapavoor (T.P) Melur North Street. , R.C Primary School,North New 3.Keelapavoor (T.P) Melur South St. , 4.Keelapavoor (T.P) Melur Road. , 1 1 Building South 5.Keelapavoor (T.P) Gurukkal Madam. , 6.Keelapavoor (T.P) Mettu Street North . , All Voters Portion,Keelapavoor. 7.Keelapavoor (T.P) Mettu St South. , 99.OVERSEAS ELECTORS OVERSEAS ELECTORS 1.Keelapavoor (T.P) Agraharam 2nd St. , 2.Keelapavoor (T.P) Agraharam 3rd Street. R.C Primary School,North New , 3.Keelapavoor (T.P) Agraharam 4th St. , 4.Keelapavoor (T.P) Sathiram Road. , 2 2 Building North All Voters 5.Keelapavoor (T.P) Surandai Road 13 Sandhu. , 6.Keelapavoor (T.P) Tamilar 14 th Portion,Keelapavoor. St. , 99.OVERSEAS ELECTORS OVERSEAS ELECTORS 1.Keelapavoor (t.p) Harijan South Street , 2.Keelapavoor (T.P) Harijan Middle St. , 3.Keelapavoor (T.P) Harijan North Street. , 4.Keelapavoor (T.P) Pillayar Kovil Soma Sundaram Hindu Primary Street. , 5.Keelapavoor (T.P) Devar South Street. , 6.Keelapavoor (T.P) Cinema 3 3 School,Western Theatre St. , 7.Keelapavoor (T.P) Chidambarapuram St. , 8.Keelapavoor T.P Water All Voters Portion,Keelapavoor Road 17.2 , 9.Keelapavoor (T.P) Asari West St. , 10.Keelapavoor (T.P) Matha Kovil St. , 11.Keelapavoor (T.P) Ramasamy Kovil Street. , 12.Keelapavoor (T.P) Kottaiyur South St. -

Annual Report - 2002-2003
LIVE TO SERVE AMAR S EVA ANNUAL REPORT - 2002-2003 SANGAM Regn.No. TSI/ 16/1981 e have completed yet another year of service in the annals of Amar Seva Sangam and W we have immense pleasure in presenting the activities that the year 2002-2003 witnessed. he year under report was unique, for the year had the distinction of Amar Seva Sangam T receiving the National Award at the hands of the President of India for the Best Institution serving the cause of the disabled. This Award coupled with the Award the Institution received from the Government of Tamilnadu in the year 2000 has indeed given not only recognition to the various activities Amar Seva Sangam is engaged in but has also given boost to it to work towards its goal with more vigor and enthusiasm. His Holiness Shri Jeyandra Saraswathi Swamigal of Kanchi Mutt awarded Mr.S.Ramakrishnan, the President of the Sangam, with Golden Medallions on the occasion of the Golden Jubilee celebrations of His Holiness having ascended the Abode of the Mutt. One of our Amar Seva Sangam's Founding members Mr.K.Chidambaram who has been the pillar of support for the organization has been awarded by Bharathi Yuve Kendra, Madurai-3 the "Best Achievement Award" during the year for his prodigious social services. He is also the Sangam's senior mason and belongs to the village of Ayikudy. Apart from extending valuable support to the Sangam since its inception, he has also been doing yeomen services to the community. The Award is a fitting recognition for his services. -
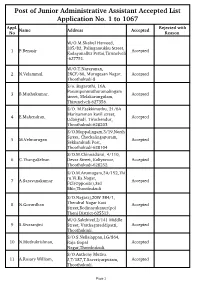
Post of Junior Administrative Assistant Accepted List Application No. 1 to 1067 Appl
Post of Junior Administrative Assistant Accepted List Application No. 1 to 1067 Appl. Rejected with Name Address Accepted No Reason W/O.M.Shahul Hameed, 205/82, Palingamukku Street, 1 P.Benasir Accepted Kadayanallur Pettai,Tirunelveli -627751. W/O.T.Narayanan, 2 N.Velammal. 2BCE/66, Murugesan Nagar, Accepted Thoothukudi-8 S/o. Bagavathi, 16A, Pasumponmuthuramalingam 3 B.Muthukumar, Accepted street, Melakarungulam, Thirunelveli-627356. S/O. M.Esakkimuthu, 21/6A Mariyamman kovil street, 4 E.Mahendran, Accepted Udangudi, Tiruchendur, Thoothukudi-628203 S/O.Muppalingam,3/19,North Street, Chockalingapuram, 5 M.Velmurugan Accepted Sekkarakudi Post, Thoothukudi-628104 S/O.M.Chinnadurai, 4/110, 6 C.ThangaSelvan Devar Street, Kaliyavoor, Accepted Thoothukudi-628252. S/O.M.Arumugam,3A/152,Thi ru.Vi.Ka.Nagar, 7 A.Saravanakumar Accepted FCI(Opposite),3rd Mile,Thoothukudi S/O.Nagaraj,20W 3B4/1, 8 N.Govardhan Thendral Nagar East Accepted Street,Bodinayakanur(po) Theni District-625513. W/O.Sakthivel,2/141 Middle 9 S.Sivaranjini Street, Varthagareddipatti, Accepted Thoothukudi. S/O.S.Nellaiappan,1G/864, 10 N.Muthukrishnan, Raja Gopal Accepted Nagar,Thoothukudi. S/O.Anthony Muthu. 11 A.Rosary William, J,7/187,T.Saveriyarpuram, Accepted Thoothukudi. Page 1 S/O.S.Seenivasagam, 7/36,Colony Street, 12 S.Premkumar, k.Velayuthapurm, Accepted Kurukkuchalai Post,Ottapidaram Taluk,Thoothukudi. D/O.Pandiyarajan,1/73,Vaniya 13 P.Gunavathy, vallam,NainarKoil Accepted Post,Paramakudi,Ramanathap uram District. D/O.K.Vilvanathan, 14 K.V.Maharasi Malathi 25/29,Toovipuram West Street Accepted No 4,Thoothukudi. S/O.P.Ragunathan, 51,Chellappa Nagar, 15 G.R.Boobalan, Madeswran Koil Accepted Street,Gobichettipalayam Post,Erode District. -

University Tirunelveli
MANONMANIAM SUNDARANAR UNIVERSITY TIRUNELVELI ACT 1990 1 AMENDMENT TO THE MANONMANIAM SUNDARANAR UNIVERSITY ACT 1990 I. The following amendment to the Act of Manonmaniam Sundaranar University has been published in the Tamil Nadu Government Gazette on Wednesday, September 18, 1991. In Section 19 of the Manonmaniam Sundaranar University Act, 1990, in the proviso to clause (b) for the words "Provided that” the following shall be substituted namely:- "Provided that a member of the Tamil Nadu Legislative Assembly shall cease to be a member of the Senate from the date on which he ceases to be a member of the Tamil Nadu Legislative Assembly: Provided further that". II. The following amendment to the Act of Manonmaniam Sundaranar University has been published in the Tamil Nadu Government Gazette on Friday, January 10, 1992. In Section 11 of the Manonmaniam Sundaranar University Act, 1990, to Sub - section (1) the following proviso shall be added, namely:- "Provided that if the Chancellor does not approve any of the persons in the panel so recommended by the Committee, he may take steps to constitute another Committee, in accordance with Sub-section (2), to give a fresh panel of three different names and shall appoint one of the persons named in the fresh panel as the Vice-Chancellor". III. The following Amendment to the Act of Manonmaniam Sundaranar University has been published in the Tamil Nadu Gazette on Friday, November 16, 2012. In Section 11 of the Manonmaniam Sundaranar University Act, 1990, in the third proviso to Sub-section (3), for the expression “Sixty – Five – years”, the expression “Seventy years” shall be substituted. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2014 [Price: Rs. 28.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 40] CHENNAI, WEDNESDAY, OCTOBER 22, 2014 Aippasi 5, Jaya, Thiruvalluvar Aandu – 2045 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 13133-3202 Notice .. 3203 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 55107. My daughter, M. Naila Begam, born on 13th October 55110. I, J. Meharunnisha, wife of Thiru M. Jamal 1999 (native district: Pudukkottai), residing at No. 1244, Mohamued, born on 31st May 1990 (native district: Kamban Nagar, Pudukkottai-622 003, shall henceforth be Madurai), residing at No. 4/303, Bama Nagar, Parasurampatti, known as M NYLA. K.Pudur, Madurai-625 007, shall henceforth be known as J. MEHAR NISHA. K. MOHAMED RABEEK. Pudukkottai, 13th October 2014. (Father.) J. MEHARUNNISHA. Madurai, 13th October 2014. 55108. My son, B. Karan, born on 6th June 1997 (native 55111. My daughter, P. Anitha, born on 11th November district: Ramanathapuram), residing at Old No. 2/172A, New 1997 (native district: Theni), residing at No. 9, Bharathiyar No. 2/192, Karmealpuram, Senpakanoor Post, Kodaikanal, East Street, Thirunagar, Madurai-625 006, shall henceforth Dindigul-624 104, shall henceforth be known as P. KARAN. -

IMPORTANT PHONE NUMBERS and EMERGENCY OPERATION CENTRE (EOC) and IT's CONTACT NUMBER Tirunelveli 0462 – 2333169 Manur 0462
IMPORTANT PHONE NUMBERS AND EMERGENCY OPERATION CENTRE (EOC) AND IT’S CONTACT NUMBER Name of the Office Office No. Collector’s Office Control Room 0462-2501032-35 Collector’s Office Toll Free No. 1077 Revenue Divisional Officer, Tirunelveli 0462-2501333 Revenue Divisional Officer, Cheranmahadevi 04634-260124 Tirunelveli 0462 – 2333169 Manur 0462 - 2485100 Palayamkottai 0462 - 2500086 Cheranmahadevi 04634 - 260007 Ambasamudram 04634- 250348 Nanguneri 04635 - 250123 Radhapuram 04637 – 254122 Tisaiyanvilai 04637 – 271001 1 TALUK TAHSILDAR Taluk Tahsildars Office No. Residence No. Mobile No. Tirunelveli 0462-2333169 9047623095 9445000671 Manoor 0462-2485100 - 9865667952 Palayamkottai 0462-2500086 - 9445000669 Ambasamudram 04634-250348 04634-250313 9445000672 Cheranmahadevi 04634-260007 - 9486428089 Nanguneri 04635-250123 04635-250132 9445000673 Radhapuram 04637-254122 04637-254140 9445000674 Tisaiyanvilai 04637-271001 - 9442949407 2 jpUney;Ntyp khtl;lj;jpy; cs;s midj;J tl;lhl;rpah; mYtyfq;fspd; rpwg;G nray;ghl;L ikaq;fspd; njhiyNgrp vz;fs; kw;Wk; ,izatop njhiyNgrp vz;fs; tpguk; fPo;f;fz;lthW ngwg;gl;Ls;sJ. Sl. Mobile No. with Details Land Line No No. Whatsapp facility 0462 - 2501070 6374001902 District EOC 0462 – 2501012 6374013254 Toll Free No 1077 1 Tirunelveli 0462 – 2333169 9944871001 2 Manur 0462 - 2485100 9442329061 3 Palayamkottai 0462 - 2501469 6381527044 4 Cheranmahadevi 04634 - 260007 9597840056 5 Ambasamudram 04634- 250348 9442907935 6 Nanguneri 04635 - 250123 8248774300 7 Radhapuram 04637 – 254122 9444042534 8 Tisaiyanvilai 04637 – 271001 9940226725 3 K¡»a Jiw¤ jiyt®fë‹ bršngh‹ v©fŸ mYtyf vz; 1. kht£l M£Á¤ jiyt®, ÂUbešntè 9444185000 2. kht£l tUthŒ mYty®, ÂUbešntè 9445000928 3. khefu fhtš Miza®, ÂUbešntè 9498199499 0462-2970160 4. kht£l fhtš f©fhâ¥ghs®, ÂUbešntè 9445914411 0462-2568025 5.