Idiopathic Inflammatory Myopathy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Percutaneous Conchotome Muscle Biopsy. a Useful Diagnostic and Assessment Tool CHRISTINA DORPH, INGER NENNESMO, and INGRID E
Percutaneous Conchotome Muscle Biopsy. A Useful Diagnostic and Assessment Tool CHRISTINA DORPH, INGER NENNESMO, and INGRID E. LUNDBERG ABSTRACT. Objective. To evaluate the diagnostic yield, performance simplicity, and safety of the percutaneous conchotome muscle biopsy technique for clinical and research purposes in an outpatient rheuma- tology clinic. Methods. Biopsies taken by rheumatologists in an outpatient clinic during 1996 and 1997 were eval- uated for histopathological and clinical diagnoses. Results. A total of 149 biopsies were performed on 122 patients. Physicians learned the method easily. Samples were of adequate size and quality to allow for diagnostics. In total 106 biopsies were taken due to different diagnostic suspicions: 24 polymyositis (PM) or dermatomyositis (DM); 43 PM, DM, or vasculitis in addition to another rheumatic condition; 19 systemic vasculitis; and 20 myalgias. Criteria for definite or probable PM/DM were fulfilled in 21 patients, 18 with positive biopsies. Thirteen patients received vasculitis as clinical diagnosis, 3 with positive biopsies. No patient with myalgia had a biopsy with inflammatory changes. Fifteen of 43 rebiopsies performed to assess disease activity had signs of active inflammation. In 48% there were changes in immuno- suppressive therapy after biopsy results. Four complications occurred; one was a serious subfascial hematoma. Conclusion. The percutaneous conchotome muscle biopsy technique gives a good size sample that allows for diagnostic evaluation and has a high yield in patients with myositis. It is a simple proce- dure, easy to learn and to perform, with a low complication rate and minimum discomfort for the patient. The method can preferably be used as a diagnostic tool and to perform repeated biopsies to assess the effect of a given therapy for both clinical and research purposes. -

Brachio-Cervical Inflammatory Myopathy with Associated Scleroderma Phenotype and Lupus Serology Andrew F
Clinical/Scientific Notes Andrew F. Gao, MD BRACHIO-CERVICAL INFLAMMATORY myopathy with the distinctive prominent B-cell infil- Philip A. Saleh, MD MYOPATHY WITH ASSOCIATED SCLERODERMA trates and endomysial MAC deposition (figure). Charles D. Kassardjian, PHENOTYPE AND LUPUS SEROLOGY The patient was treated with high-dose IV meth- MD ylprednisolone for 4 days, followed by 1 mg/kg oral Ophir Vinik, MD Brachio-cervical inflammatory myopathy (BCIM) is prednisone and a gradual taper and azathioprine. David G. Munoz, MD a unique clinicopathologic entity characterized by She received monthly IV immunoglobulin. At 1 neck and upper extremity weakness with relative spar- 5-month follow-up, strength improved 4 /5 in the Neurol Neuroimmunol affected muscles. The CK level declined to 167 IU/L. Neuroinflamm ing of lower extremities and commonly associated 2018;5:e410; doi: 10.1212/ with connective tissue diseases or myasthenia gravis Dysphagia improved, and G-tube feeding could be NXI.0000000000000410 and serum autoantibodies (e.g., antinuclear antibody discontinued with resumption of solid oral diet. [ANA], anti–double stranded DNA [dsDNA], and Discussion. Our case is a prototypical example of anti–acetylcholine receptor).1 Muscle pathology is BCIM, demonstrating the clinicopathologic features distinctive, with prominent B-cell infiltrates and en- first described by Pestronk.1 They reported that the domysial membrane attack complex (MAC; C5b-9) most commonly associated conditions were myasthe- deposition. Despite the detailed original series, there nia gravis (40%) and rheumatoid arthritis (20%). have been no subsequent reports (besides abstracts2,3) Muscle biopsies showed extensive inflammatory infil- demonstrating the full clinicopathologic features of trates with at least 1 prominent CD201 B-cell focus, BCIM. -

Dropped Head Syndrome Due to Neuromuscular Disorders: Clinical
Neurology International 2019; volume 11:8198 Dropped head syndrome due inflammatory polyneuropathy (CIDP),11 to neuromuscular disorders: neuromuscular causes include myasthenia Correspondence: Ahmet Z. Burakgazi, gravis (MG),12-14 Lambert-Eaton myasthe- Neuroscience Section, Department of Clinical manifestation and nia syndrome (LEMS),15 muscular causes Medicine, Virginia Tech Carilion School of evaluation includes primary inflammatory such as Medicine, 3 Riverside Circle, Roanoke, VA polymyositis,16 scleromyositis,17,18 isolated 24016, USA. inflammatory axial myopathy,19 primary Tel.: +1.540-521-4592. Ahmet Z. Burakgazi, Perry K. E-mail: [email protected] Richardson, Mohammad Abu-Rub non-inflammatory such as nemaline myopa- 20-22 thy, mitochondrial myopathy, congeni- Key words: Dropped head syndrome, neuro- Virginia Tech Carilion School of 23 24 tal myopathy, FSHD, and isolated neck muscular disease. Medicine, Roanoke, VA, USA extensor myopathy (INEM).19 Contributions: the authors contributed equally. Conflict of interest: the authors declare no Abstract General approach: clinical mani- potential conflict of interest. festation and evaluation In this article, we discuss the clinical Funding: none. approach to patients with dropped head syn- DHS occurs as a result of weakness of drome and identify the various neuromus- posterior neck muscles. It usually disap- Received for publication: 11 June 2019. cular causes of dropped head syndrome pears with supine position. The common Accepted for publication: 18 June 2019. including muscle, neuromuscular junction, chief complaints are “chin on the chest” and This work is licensed under a Creative peripheral nerve and motor neuron etiolo- “difficulty maintaining a forward gaze”. It gies. We aim to increase awareness of Commons Attribution NonCommercial 4.0 may contribute to dysphagia and has cos- License (CC BY-NC 4.0). -

Diagnosis and Treatment of Dermatomyositis-Systemic Lupus
Diagnosis and Treatment of Dermatomyositis-Systemic Lupus Erythematosus Overlap Syndrome Preston Williams1; Benjamin McKinney, MD2 1Texas A&M College of Medicine; 2Baylor University Medical Center Family Medicine Residency Introduction Case Description Discussion Dermatomyositis is an autoimmune condition classically A punch biopsy of her rash showed atrophic epithelium with This case of overlap syndrome between dermatomyositis and characterized by symmetric proximal muscle weakness, dyskeratotic keratinocytes, vacuolar interface changes, superficial systemic lupus erythematosus presents a rare but important inflammatory muscle changes, and dermatologic abnormalities.1 perivascular and lichenoid inflammation, and pigment challenge to the primary care physician. Our patient presented Several studies have shown that the inflammatory myopathies, incontinence consistent with systemic lupus erythematosus initially with arthralgias and fatigue, symptoms more such as dermatomyositis, commonly overlap with other (SLE). The patient was started on prednisone 40 mg daily for a 2- characteristic of systemic lupus erythematosus. However, these connective tissue disorders, significantly complicating the week taper to 10 mg and hydroxychloroquine 200 mg daily with symptoms were followed by a facial rash that involved the diagnosis.2 The reported incidence of overlap syndrome ranges marked improvement in symptoms. Further lab work-up was nasolabial folds and periorbital regions more in line with from 11% to 40% in patients diagnosed with significant -
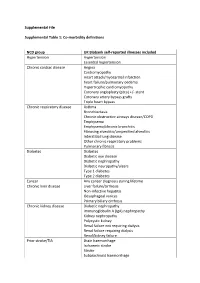
Supplemental File Supplemental Table 1: Co-Morbidity Definitions
Supplemental File Supplemental Table 1: Co-morbidity definitions NCD group UK Biobank self-reported illnesses included Hypertension Hypertension Essential hypertension Chronic cardiac disease Angina Cardiomyopathy Heart attack/myocardial infarction heart failure/pulmonary oedema Hypertrophic cardiomyopathy Coronary angioplasty (ptca) +/- stent Coronary artery bypass grafts Triple heart bypass Chronic respiratory disease Asthma Bronchiectasis Chronic obstructive airways disease/COPD Emphysema Emphysema/chronic bronchitis Fibrosing alveolitis/unspecified alveolitis Interstitial lung disease Other chronic respiratory problems Pulmonary fibrosis Diabetes Diabetes Diabetic eye disease Diabetic nephropathy Diabetic neuropathy/ulcers Type 1 diabetes Type 2 diabetes Cancer Any cancer diagnosis during lifetime Chronic liver disease Liver failure/cirrhosis Non-infective hepatitis Oesophageal varices Primary biliary cirrhosis Chronic kidney disease Diabetic nephropathy Immunoglobulin A (IgA) nephropathy Kidney nephropathy Polycystic kidney Renal failure not requiring dialysis Renal failure requiring dialysis Renal/kidney failure Prior stroke/TIA Brain haemorrhage Ischaemic stroke Stroke Subarachnoid haemorrhage Transient ischaemic attack (TIA) Other neurology disease Cerebral palsy Epilepsy Motor neurone disease Multiple sclerosis Myasthenia gravis Parkinson’s disease Psychiatric disease Depression Mania/bipolar disorder/manic depression Postnatal depression Schizophrenia Chronic inflammatory and Ankylosing spondylitis autoimmune rheumatic disease -

A Case of Dermatomyositis with Secondary Sjögren's Syndrome
162 A Case of Dermatomyositis with Secondary Sjögren’s Syndrome- Diagnosis with Follow-up Study of Technetium-99m Pyrophosphate Scintigraphy Ching-Tang Huang1, Ying-Chu Chen1, Chingtsai Lin2, Yu-Chun Hsiao3, Lai-Fa Sheu4, Min-Chien Tu1 Abstract Purpose: To report a case of dermatomyositis (DM) with secondary Sjögren’s syndrome (SS) and propose the clinical application of technetium-99m pyrophosphate (99mTc-PYP) scan. Case Report: A 50-year-old woman had progressive proximal muscle weakness of bilateral thighs, myalgia, tea-colored urine, and exercise intolerance for 6 months. Physical examination showed malar rash, V-sign, periungual erythema, and mechanic hands. Neurological assessment showed symmetric pelvic-girdle weakness, myopathic face, waddling gait, but preserved deep tendon reflex and sensory functions. DM was diagnosed on the basis of typical rashes and serum creatinine kinase elevation (7397 IU/L). Aside from myopathic symptoms, dry eye and mouth were reported. Thorough autoantibody searches showed positive anti-SSA/Ro antibody (198 U/ml). Both Schirmer's test and sialoscintigraphy were positive, leading secondary SS as diagnosis. Initial 99mTc-PYP scan revealed increased radiouptake in the muscles of bilateral thighs, compatible with clinical assessment. Follow- up scan three months later shows abnormal but attenuated radiouptake at bilateral thighs, in the presence of nearly-complete clinical recovery. Conclusion: DM with secondary SS in adult is a unique disease entity, with predominantly myopathic symptoms and satisfactory therapeutic response as its characteristics. Our serial muscle imaging studies suggest that 99mTc-PYP scan is at once anatomically-specific and persistently-sensitive to microstructural damages within inflammatory muscles, enabling clinician to monitor disease activity and therapeutic response. -

Facioscapulohumeral Muscular Dystrophy
Lab Management Guidelines V1.0.2020 Genetic Testing for Facioscapulohumeral Muscular Dystrophy MOL.TS.290.A v1.0.2020 Introduction Facioscapulohumeral Muscular Dystrophy testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedure addressed by this guideline Procedure code D4Z4 region (FSHMD1A) deletion 81404 analysis D4Z4 region (FSHMD1A) methylation 81479 analysis FSHMD1 characterization of 4qA/4qB 81404 haplotypes SMCHD1 sequencing 81479 SMCHD1 deletion/duplication analysis 81479 What is Facioscapulohumeral Muscular Dystrophy Definition Facioscapulohumeral muscular dystrophy (FSHD) is both a genetic & epigenetic condition characterized by progressive muscle weakness involving facial, scapular, and humeral muscle groups early, and pelvic and peroneal muscle groups later.1,2 There are two types of FSHD (FSHD1 and FSHD2) that are clinically identical, but distinguished by their different genetic causes. Incidence and Prevalence Prevalence is estimated between 4-10 per 100,000. Approximately 95% of FSHD cases are FSHD1; the remaining cases are FSHD2.3 © 2020 eviCore healthcare. All Rights Reserved. 1 of 9 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V1.0.2020 Symptoms Signs and symptoms can begin anytime between childhood and adulthood, but typical manifestations -

Document Downloaded from Day 24/07
Document downloaded from http://www.elsevier.es, day 27/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Document downloaded from http://www.elsevier.es, day 27/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Supplementary Table. Search strategy PUBMED #P1 "Idiopathic inflammatory myopathy" OR "autoimmune myopathy" OR myositis OR 205200 myopathy OR dermatomyositis OR "clinically amyopathic dermatomyositis" OR "amyopathic dermatomyositis" OR "hypomyopathic dermatomyositis" OR "juvenile dermatomyositis" OR "clinically amyopathic juvenile dermatomyositis" OR dermatopolymyositis OR polymyositis OR "immune-mediated necrotizing myopathy" OR "inclusion body myositis" OR "overlap myositis" OR "anti-synthetase syndrome" OR "cancer-associated myositis" #P2 Africa [MeSH] OR Africa [tw] OR Algeria [tw] OR Angola [tw] OR Benin [tw] OR 568425 Botswana [tw] OR Burkina Faso[tw] OR Burundi [tw] OR Cameroon [tw] OR Canary Islands [tw] OR Cape Verde [tw] OR Central African Republic [tw] OR Chad [tw] OR Comoros [tw] OR Congo [tw] OR Democratic Republic of Congo [tw] OR Djibouti [tw] OR Egypt [tw] OR Equatorial Guinea [tw] OR Eritrea [tw] OR Ethiopia [tw] OR Gabon [tw] OR Gambia [tw] OR Ghana [tw] OR Guinea [tw] OR Guinea Bissau [tw] OR Ivory Coast [tw] OR Cote d’Ivoire [tw] OR Kenya [tw] OR Lesotho [tw] OR Liberia [tw] OR Libya [tw] OR Libia [tw] OR Madagascar [tw] OR Malawi [tw] OR Mali [tw] OR Mauritania [tw] OR -

For Peer Review Hudaifa Alani¹, Julian R
Scandinavian Journal of Rheumatology Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren’s syndrome (secondary Sjögren’s syndrome)For Peer focusing Review on autoimmune rheumatic diseases Journal: Scandinavian Journal of Rheumatology Manuscript ID SJR-2016-0514.R2 Manuscript Type: Article Date Submitted by the Author: 18-Apr-2017 Complete List of Authors: Alani, Hudaifa; Kettering General Hospital NHS Foundation Trust Henty, Julian; University College London Thompson, Nicolyn; University College London Jury, EC; University College London Ciurtin, Coziana; University College London, Department of Rheumatology secondary Sjogren's syndrome, epidemiology, Meta-analysis, systematic Keywords: review, polyautoimunity Scandinavian Journal of Rheumatology Page 1 of 71 Scandinavian Journal of Rheumatology Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren’s syndrome (secondary Sjögren’s syndrome) focusing on autoimmune rheumatic diseases Submission type: article For Peer Review Short title: Polyautoimmunity in Sjögren's syndrome Authors: Hudaifa Alani¹, Julian R. Henty², Nicolyn L Thompson³, Elizabeth Jury³ and Coziana Ciurtin³* ¹Kettering General Hospital, NN16 8UZ, Kettering, United Kingdom ²Department of Medical Physics, University College London, London, United Kingdom ³Department of Rheumatology, University College London, London, United Kingdom Scandinavian Journal of Rheumatology Scandinavian Journal of Rheumatology Page 2 of 71 *Corresponding author: Dr. Coziana Ciurtin, PhD, FRCP, Department of Rheumatology, University College London, 250 Euston Road, London, NW1 2PG, email: [email protected], phone: +44(0)2034479035, fax: +44(0)20344797268 . For Peer Review Scandinavian Journal of Rheumatology Page 3 of 71 Scandinavian Journal of Rheumatology Abstract Objective: The epidemiology of polyautoimmunity in Sjögren's syndrome (secondary Sjögren's syndrome - sSS) is not well-defined and was not investigated before using a systematic approach. -

Approach to Patients with Neuromuscular Disorders
Approach to Patients With Neuromuscular Disorders Anthony A. Amato, MD Chief, Neuromuscular Division and Director, Clinical Neurophysiology Laboratory Brigham and Women’s Hospital Associate Professor of Neurology Harvard Medical School Boston, Massachusetts INTRODUCTION a true sensory level is not evident in GBS but should be present in transverse myelitis and other structural abnormalities involving the The approach to patients with neuromuscular disorders is chal- spinal cord. lenging. As in other neurological diseases the key to arriving at the correct diagnosis is careful localization of the lesion. Weakness can The presence of motor and sensory symptoms and signs are helpful be the result of central lesions (brain or spinal cord processes—e.g., in distinguishing peripheral neuropathies from anterior horn cell brainstem infarct, central pontine myelinolysis, transverse myelopa- disorders, myopathies, and neuromuscular junction disorders. thy), anterior horn cell disease (e.g., amyotrophic lateral sclerosis However, some types of peripheral neuropathy are predominantly [ALS], poliomyelitis), peripheral neuropathy (e.g., Guillain-Barré or purely motor and thus can be difficult to distinguish these other syndrome [GBS]), neuromuscular junction defects (botulism, disease processes. Most neuropathies are associated with distal Lambert-Eaton myasthenic syndrome [LEMS], myasthenia gravis greater than proximal weakness. However, significant proximal [MG]), or myopathic disorders. The most important aspect of as- weakness can be seen in certain peripheral neuropathies (e.g., GBS, sessing individuals with neuromuscular disorders is taking a thor- chronic inflammatory demyelinating polyneuropathy [CIDP]). ough history of the patient’s symptoms, disease progression, and Further, although usually associated with proximal weakness, past medical and family history as well as performing a detailed certain myopathies and rarely even neuromuscular junction disor- neurologic examination. -

Chronic Intestinal Pseudo-Obstruction in Systemic Lupus Erythematosus Gut: First Published As 10.1136/Gut.43.1.117 on 1 July 1998
Gut 1998;43:117–122 117 Chronic intestinal pseudo-obstruction in systemic lupus erythematosus Gut: first published as 10.1136/gut.43.1.117 on 1 July 1998. Downloaded from G Perlemuter, S Chaussade, B Wechsler, P Cacoub, M Dapoigny, A Kahan, P Godeau, D Couturier Abstract enteric nerves, or the visceral autonomic nerv- Background/Aims—Chronic intestinal ous system. It may be the primary disease or it pseudo-obstruction (CIPO) reflects a dys- may be secondary to a recognised underlying function of the visceral smooth muscle or disease and then defined as secondary CIPO. the enteric nervous system. Gastrointesti- The causes of secondary CIPO are numerous nal manifestations are common in systemic and involve the nervous, endocrine, and meta- lupus erythematosus (SLE) but CIPO has bolic systems, intra-abdominal inflammation, not been reported. Features of CIPO are infiltrative and connective tissue diseases, and reported in five patients with SLE. drug induced states.2 Mild gastrointestinal Methods—From 1988 to 1993, five patients manifestations are common in systemic lupus with SLE or SLE-like syndrome were hos- erythematosus (SLE). Nausea, vomiting, diar- pitalised for gastrointestinal manometric rhoea, and abdominal pain are found in more studies. CIPO was the onset feature in two than 50% of these patients.3 Motility disorders cases. Antroduodenal manometry (three of the gastrointestinal tract are less frequent. hours fasting, two hours fed) was per- CIPO is rare in SLE. Since our first published formed in all patients, and oesophageal case,4 four other patients with SLE have been manometry in four. hospitalised in our department with a diagnosis Results—Intestinal hypomotility associ- of CIPO, confirmed by manometry. -

Dermatomyositis and Polymyositis
ARTIGO DE REVISÃO Dermatomiosite e polimiosite: da imunopatologia à imunoterapia (imunobiológicos) Samuel Katsuyuki Shinjo1, Fernando Henrique Carlos de Souza2, Julio Cesar Bertacini de Moraes2 RESUMO As miopatias infl amatórias idiopáticas (MII), das quais fazem parte a dermatomiosite (DM) e a polimiosite (PM), são doenças sistêmicas crônicas associadas a alta morbidade e incapacidade funcional. O tratamento atual baseia-se na corticoterapia e no uso de imunossupressores, porém uma parcela considerável dos pacientes é refratária à terapia tradicional. Isso tem levado à tentativa de uso de imunobiológicos nesses pacientes, tendo por fundamento a fi siopatogênese das MII. Do ponto de vista imunopatológico, há diferenças entre PM e DM: a primeira está mais relacionada à imunidade celular, enquanto na segunda o papel humoral parece mais importante. Em ambas, porém, são descritas concentrações elevadas de interleucinas pró-infl amatórias (TNF, IL-1, IL-6) e aumento da expressão de moléculas relacionadas à coestimulação dos linfócitos T – nessas condições, parece racional o uso da terapia biológica. Considerando os imunobiológicos disponíveis, são escassos os dados de trabalhos abertos na literatura, compostos principalmente por séries e relatos de casos. Os bloqueadores do TNF apresentam resultados confl itantes sem evidência de boa resposta ao tratamento. A terapia anti-CD20 possui os resultados mais promissores. É extremamente escassa a informação sobre o bloqueio da coestimulação do linfócito T e a terapia anti- IL-6, que impede qualquer consideração. Dessa maneira, o uso de imunobiológicos em MII ainda permanece como fronteira a ser explorada. A terapia biológica pode ter papel relevante no tratamento das MII refratárias à terapia convencional; no entanto, novos estudos prospectivos com base em parâmetros objetivos de resposta ao tratamento são necessários.