Evaluating the Effects of Hydrogeologic Processes on the Fate of Salinity and Halogens in Headwater Catchments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Section 5.4.3: Risk Assessment – Flood
SECTION 5.4.3: RISK ASSESSMENT – FLOOD 5.4.3 FLOOD This section provides a profile and vulnerability assessment for the flood hazard. HAZARD PROFILE This section provides hazard profile information including description, extent, location, previous occurrences and losses and the probability of future occurrences. Description Floods are one of the most common natural hazards in the U.S. They can develop slowly over a period of days or develop quickly, with disastrous effects that can be local (impacting a neighborhood or community) or regional (affecting entire river basins, coastlines and multiple counties or states) (Federal Emergency Management Agency [FEMA], 2006). Most communities in the U.S. have experienced some kind of flooding, after spring rains, heavy thunderstorms, coastal storms, or winter snow thaws (George Washington University, 2001). Floods are the most frequent and costly natural hazards in New York State in terms of human hardship and economic loss, particularly to communities that lie within flood prone areas or flood plains of a major water source. The FEMA definition for flooding is “a general and temporary condition of partial or complete inundation of two or more acres of normally dry land area or of two or more properties from the overflow of inland or tidal waters or the rapid accumulation of runoff of surface waters from any source (FEMA, Date Unknown).” The New York State Disaster Preparedness Commission (NYSDPC) and the National Flood Insurance Program (NFIP) indicates that flooding could originate from one -

Pearly Mussels in NY State Susquehanna Watershed Paul H
Pearly mussels in NY State Susquehanna Watershed Paul H. Lord, Willard N. Harman & Timothy N. Pokorny Introduction Preliminary Results Discussion Pearly mussels (unionids) New unionid SGCN identified • Mobile substrates appear exacerbated endangered native mollusks in Susquehanna River Watershed by surge stormwater inputs • Life cycle complex • Eastern Pearlshell (Margaritifera margaritifera) - made worse by impervious surfaces - includes fish parasitism -- in Otselic River headwaters • Unionids impacted - involves watershed quality parameters Historical SGCN found in many locations by ↓O2, siltation, endocrine disrupting chemicals • 4 Species of Greatest Conservation Need • Regularly downstream of extended riffle - from human watershed use (SGCN) historically found • Require minimally mobile substrates • River location consistency with old maps in NY State Susquehanna Watershed • No observed wastewater treatment plant impact associated with ↑ unionids - Brook Floater (Alasmidonta varicosa) -adult unionids more easily observed - Green Floater (Lasmigona subviridis) Table 1. NYSDEC freshwater pearly mussel “species of greatest conservation need” (SGCN) observed in the Upper Susquehanna from kayaks - Yellow Lamp Mussel (Lampsilis cariosa) Watershed while mapping and searching rivers in the summers of 2008 Elktoe -Elktoe (Alasmidonta marginata) and 2009. Brook Floater = Alasmidonta varicosa; elktoe = Alasmidonta • Prior sampling done where convenient marginata; green floater = Lasmigona subviridis; yellow lamp mussel = - normally at intersection -

Comprehensive Plan (Draft)
Town of Cortlandville Comprehensive Plan Prepared for the Cortlandville Town Board December 2020 Town of Cortlandville, NY December 2020 Comprehensive Plan Draft Acknowledgements The Town of Cortlandville would like to thank the Comprehensive Plan committee for their efforts and hard work during the preparation of this important document. The Town would also like to thank Town officials and employees who willingly answered questions and provided data. John Proud, former Town Board Member who served as the Town Board liaison during his tenure and remains as a technical advisor to the Committee deserves special recognition. His willingness to answer questions, provide additional information or direct the committee to additional information sources and his deep knowledge of the Town has been an asset to the Committee. Town Board Tom Williams, Supervisor Ted Testa Jay Cobb Doug Withey Jeff Guido Prior Town Board Richard Tupper John Proud Randolph Ross Comprehensive Plan Committee Nasrin Parvizi, Chair Forrest Earl Ann Hotchkin Pam Jenkins David Yaman Town of Cortlandville, NY December 2020 Comprehensive Plan Draft Table of Contents Page Executive Summary E-1 Chapter 1 Introduction Comprehensive Plan Process 1-1 Legislative Authority 1-3 Public Participation 1-3 Chapter 2 Cortlandville Today Historical Background 2-1 Present Day 2-2 Where Are We? 2-4 Previous Planning Activities 2-6 Chapter 3 Cortlandville’s Vision Vision 3-1 Goals and Objectives 3-2 Chapter 4 Plan Recommendations Growth Management and Land Use 4-1 Infrastructure 4-8 Transportation -

Flood Event of 3/4/1964 - 3/7/1964
Flood Event of 3/4/1964 - 3/7/1964 Chemung Site Flood Stage Date Crest Flow Category Basin Stream County of Gage County of Forecast Point Campbell 8.00 3/5/1964 8.45 13,200 Minor Chemung Cohocton River Steuben Steuben Chemung 16.00 3/6/1964 20.44 93,800 Moderate Chemung Chemung River Chemung Chemung Corning 29.00 3/5/1964 30.34 -9,999 Moderate Chemung Chemung River Steuben Steuben Elmira 12.00 3/6/1964 15.60 -9,999 Moderate Chemung Chemung River Chemung Chemung Lindley 17.00 3/5/1964 18.48 37,400 Minor Chemung Tioga River Steuben Steuben Delaware Site Flood Stage Date Crest Flow Category Basin Stream County of Gage County of Forecast Point Walton 9.50 3/5/1964 13.66 15,800 Minor Delaware West Branch Delaware Delaware Delaware River James Site Flood Stage Date Crest Flow Category Basin Stream County of Gage County of Forecast Point Lick Run 16.00 3/6/1964 16.07 25,900 Minor James James River Botetourt Botetourt Juniata Site Flood Stage Date Crest Flow Category Basin Stream County of Gage County of Forecast Point Spruce Creek 8.00 3/5/1964 8.43 4,540 Minor Juniata Little Juniata River Huntingdon Huntingdon Created On: 8/16/2016 Page 1 of 4 Main Stem Susquehanna Site Flood Stage Date Crest Flow Category Basin Stream County of Gage County of Forecast Point Towanda 16.00 3/6/1964 23.63 174,000 Moderate Upper Main Stem Susquehanna River Bradford Bradford Susquehanna Wilkes-Barre 22.00 3/7/1964 28.87 180,000 Moderate Upper Main Stem Susquehanna River Luzerne Luzerne Susquehanna North Branch Susquehanna Site Flood Stage Date Crest Flow Category -

Lisle Flood Damage Reduction Project
LISLE FLOOD DAMAGE REDUCTION PROJECT Department of Environmental Conservation Operated and Maintained by: New York State Region 7 Counties: Broome, Cayuga, Chenango, Cortland, Madison, Onondaga, Oswego, Tioga, Tompkins PROJECT LOCATION The Village of Lisle, Broome County, New York, is located on the right bank of the Tioughnioga River, 11 miles above its confluence with the Chenango River in the upper Susquehanna River Basin. Page 1 of 6 Lisle Flood Damage Reduction Project PROJECT DESCRIPTION The improvement consists of the following work: • The relocation of about 3,000 feet of the Dudley Creek channel, extending from 1,200 feet west of the intersection of the Cortland and Main Streets to the confluence with the Tioughnioga River. • The realignment of 5,700 feet of the Tioughnioga River channel east of the Village. • Construction of about 4,150 feet of earth levee and 970 feet of concrete wall on the right bank of Dudley Creek and the Tioughnioga River extending from Main Street on the west end of the village to the railroad crossing on River Street. • A stoplog structure for the railroad through the levee. • The relocation of about 1,600 feet of Cortland Street. • Construction of railroad and highway bridges over the relocated Dudley Creek • The construction of appurtenant drainage structures. AUTHORIZATION By enactment of the Flood Control Act approved June 22nd, 1936 (Public Law No. 738, 74th Congress), Congress authorized “Construction of detention reservoirs and related flood- control works for protection of Binghamton, Hornell, Corning, and other towns in New York and Pennsylvania in accordance with plans approved by the Chief of Engineers…” The flood- protection works at Lisle, Whitney Point Village, and Oxford, New York, constructed under that authority, as amended by the Flood Control Act approved June 28th, 1938 (Public Law No 761, 75th Congress, 3rd session), are a part of the comprehensive plan for flood control in the upper Susquehanna River watershed in southern New York and eastern Pennsylvania. -

Susquehanna Riyer Drainage Basin
'M, General Hydrographic Water-Supply and Irrigation Paper No. 109 Series -j Investigations, 13 .N, Water Power, 9 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR HYDROGRAPHY OF THE SUSQUEHANNA RIYER DRAINAGE BASIN BY JOHN C. HOYT AND ROBERT H. ANDERSON WASHINGTON GOVERNMENT PRINTING OFFICE 1 9 0 5 CONTENTS. Page. Letter of transmittaL_.__.______.____.__..__.___._______.._.__..__..__... 7 Introduction......---..-.-..-.--.-.-----............_-........--._.----.- 9 Acknowledgments -..___.______.._.___.________________.____.___--_----.. 9 Description of drainage area......--..--..--.....-_....-....-....-....--.- 10 General features- -----_.____._.__..__._.___._..__-____.__-__---------- 10 Susquehanna River below West Branch ___...______-_--__.------_.--. 19 Susquehanna River above West Branch .............................. 21 West Branch ....................................................... 23 Navigation .--..........._-..........-....................-...---..-....- 24 Measurements of flow..................-.....-..-.---......-.-..---...... 25 Susquehanna River at Binghamton, N. Y_-..---...-.-...----.....-..- 25 Ghenango River at Binghamton, N. Y................................ 34 Susquehanna River at Wilkesbarre, Pa......_............-...----_--. 43 Susquehanna River at Danville, Pa..........._..................._... 56 West Branch at Williamsport, Pa .._.................--...--....- _ - - 67 West Branch at Allenwood, Pa.....-........-...-.._.---.---.-..-.-.. 84 Juniata River at Newport, Pa...-----......--....-...-....--..-..---.- -

Upper Susquehanna River Basin Flood Damage Reduction Study
Final Fish and Wildlife Coordination Act Report Upper Susquehanna Comprehensive Flood Damage Reduction Study Prepared For: U.S. Army Corps of Engineers Prepared By: Department of the Interior U.S. Fish and Wildlife Service New York Field Office Cortland, New York Preparers: Anne Secord & Andy Lowell New York Field Office Supervisor: David Stilwell February 2019 EXECUTIVE SUMMARY Flooding in the Upper Susquehanna watershed of New York State frequently causes damage to infrastructure that has been built within flood-prone areas. This report identifies a suite of watershed activities, such as urban development, wetland elimination, stream alterations, and certain agricultural practices that have contributed to flooding of developed areas. Structural flood control measures, such as dams, levees, and floodwalls have been constructed, but are insufficient to address all floodwater-human conflicts. The U.S. Army Corps of Engineers (USACE) is evaluating a number of new structural and non-structural measures to reduce flood damages in the watershed. The New York State Department of Environmental Conservation (NYSDEC) is the “local sponsor” for this study and provides half of the study funding. New structural flood control measures that USACE is evaluating for the watershed largely consist of new levees/floodwalls, rebuilding levees/floodwalls, snagging and clearing of woody material from rivers and removing riverine shoals. Non-structural measures being evaluated include elevating structures, acquisition of structures and property, relocating at-risk structures, developing land use plans and flood proofing. Some of the proposed structural measures, if implemented as proposed, have the potential to adversely impact riparian habitat, wetlands, and riverine aquatic habitat. In addition to the alternatives currently being considered by the USACE, the U.S. -

Upper Susquehanna Subbasin Survey: a Water Quality and Biological Assessment, June – September 2007
Upper Susquehanna Subbasin Survey: A Water Quality and Biological Assessment, June – September 2007 The Susquehanna River Basin Commission (SRBC) conducted a water quality and biological survey of the Upper Susquehanna Subbasin from June to September 2007. This survey is part of SRBC’s Subbasin Survey Program, which is funded in part by the United States Environmental Protection Agency (USEPA). The Subbasin Survey Program consists of two- year assessments in each of the six major subbasins (Figure 1) on a rotating schedule. This report details the Year-1 survey, which consists of point-in-time water chemistry, macroinvertebrate, and habitat data collection and assessments of the major tributaries and areas of interest throughout the Upper Susquehanna Subbasin. The Year-2 survey will be conducted in the Tioughnioga River over a one-year time period beginning in summer 2008. The Year-2 survey is part of a larger monitoring effort associated with an environmental restoration effort at Whitney Point Lake. Previous SRBC surveys of the Upper Susquehanna Subbasin were conducted in 1998 (Stoe, 1999) and 1984 (McMorran, 1985). Subbasin survey information is used by SRBC staff and others to: • evaluate the chemical, biological, and habitat conditions of streams in the basin; • identify major sources of pollution and lengths of streams impacted; • identify high quality sections of streams that need to be protected; • maintain a database that can be used to document changes in stream quality over time; • review projects affecting water quality in the basin; and • identify areas for more intensive study. Description of the Upper Susquehanna Subbasin The Upper Susquehanna Subbasin is an interstate subbasin that drains approximately 4,950 square miles of southcentral New York and a small portion of northeastern Pennsylvania. -

The New York State Flood of July 1935
Please do not destroy or throw away this publication. If you have no further use for it write to the Geological Survey at Washington and ask for a frank to return it UNITED STATES DEPARTMENT OF THE INTERIOR Harold L. Ickes, Secretary GEOLOGICAL SURVEY W. C. Mendenhall, Director Water-Supply Paper 773 E THE NEW YORK STATE FLOOD OF JULY 1935 BY HOLLISTER JOHNSON Prepared in cooperation with the Water Power and Control Commission of the Conservation Department and the Department of Public Works, State of New York Contributions to the hydrology of the United States, 1936 (Pages 233-268) UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1936 For sale by the Superintendent of Documents, Washington, D. C. -------- Price 15 cents CONTENTS Page Introduction......................................................... 233 Acknowledgments...................................................... 234 Rainfall,............................................................ 235 Causes.......................................................... 235 General features................................................ 236 Rainfall records................................................ 237 Flood discharges..................................................... 246 General features................................................ 246 Field work...................................................... 249 Office preparation of field data................................ 250 Assumptions and computations.................................... 251 Flood-discharge records........................................ -
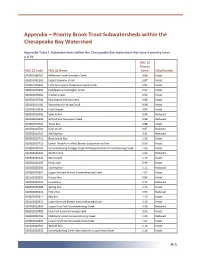
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

A Strategy for Removing Or Mitigating Dams in New York State and Lessons Learned in the Upper Susquehanna Watershed
A Strategy for Removing or Mitigating Dams in New York State and Lessons Learned in the Upper Susquehanna Watershed May 2008 New York State Department of Environmental Conservation A Nonpoint Source Coordinating Committee – Hydrologic and Habitat Modification Workgroup Publication 625 Broadway Albany, New York 12233 Prepared by: U.S. Fish and Wildlife Service 3817 Luker Road Cortland, New York 13045 NYSDEC Contract with NYSARC (C302276 Task C-6a USFWS) DEDICATION This report is dedicated to U.S. Fish and Wildlife Service Biologist Dave Bryson, who was the principal investigator for this project through 2006 but unexpectedly passed away during its development. His foresight in promoting fish passage and aquatic ecosystem restoration throughout New York State will be remembered by many. His passion for environmental protection and the art of fishing inspired us all. We will miss him. ii Foreword This report was prepared under contract for the New York State Department of Environmental Conservation (NYSDEC) through collaboration of members of the Hydrologic and Habitat Modification Workgroup (HHM), which is chaired by the NYSDEC Division of Water’s Nonpoint Source Management Section Chief. The guidance is for stream professionals, as well as individuals, agencies, and communities that have interests in evaluating dams to identify opportunities for improving fish passage and restoring river dynamics. Acknowledgements Principal Author/Principal Anne Secord, U.S. Fish and Wildlife Service (USFWS), Cortland, NY Investigator (2006-2008) Tele: -

Whitney Point Adaptive Management Plan a Five�Year Summary Luanne Steffy, Aquatic Ecologist December 2013
Whitney Point Adaptive Management Plan A Five-Year Summary Luanne Steffy, Aquatic Ecologist December 2013 Whitney Point Lake is a 1,200-acre U.S. Army Corps of Engineers (USACE) reservoir located on the Otselic River in Broome County, New York (Figure 1), that was completed in 1942 primarily for flood control purposes. Starting in mid-1990s, the Susquehanna River Basin Commission (Commission), USACE, and New York State Department of Environmental Conservation (NYSDEC) began discussions about a water management and environmental restoration project in Whitney Point Lake that would allow for water storage in the lake to be used for low flow augmentation releases. Over the following decade, discussions, negotiations, and planning continued until in 2008, a lake modification and environmental restoration plan was completed. This agreement called for not only the elimination of winter drawdown, replaced by a maintained year-round water level in Whitney Point Lake, but also provided for environmental releases from the lake to augment low flow conditions downstream in the Otselic, Tioughnioga, Chenango, and, ultimately, Susquehanna Rivers. Other supplemental improvements were also made to the lake and surrounding park area including constructed wetlands, wildlife habitat improvements, and updated recreational facilities. In order to monitor the ecological ramifications of the lake modifications and restoration plan, a five-year Adaptive Management Plan (AMP) was also established in 2008 as part of the environmental restoration plan which was to be carried out from 2009-2013. The long-term goal of the monitoring portion of the AMP was to document potential impacts of flow augmentation on aquatic communities in Whitney Point Lake and the surrounding watersheds.