Evidence of the Standing Committee on Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dealing with Crisis
Briefing on the New Parliament December 12, 2019 CONFIDENTIAL – FOR INTERNAL USE ONLY Regional Seat 8 6 ON largely Flip from NDP to Distribution static 33 36 Bloc Liberals pushed out 10 32 Minor changes in Battleground B.C. 16 Liberals lose the Maritimes Goodale 1 12 1 1 2 80 10 1 1 79 1 14 11 3 1 5 4 10 17 40 35 29 33 32 15 21 26 17 11 4 8 4 2015 2019 2015 2019 2015 2019 2015 2019 2015 2019 2015 2019 BC AB MB/SK ON QC AC Other 2 Seats in the House Other *As of December 5, 2019 3 Challenges & opportunities of minority government 4 Minority Parliament In a minority government, Trudeau and the Liberals face a unique set of challenges • Stable, for now • Campaign driven by consumer issues continues 5 Minority Parliament • Volatile and highly partisan • Scaled back agenda • The budget is key • Regulation instead of legislation • Advocacy more complicated • House committee wild cards • “Weaponized” Private Members’ Bills (PMBs) 6 Kitchen Table Issues and Other Priorities • Taxes • Affordability • Cost of Living • Healthcare Costs • Deficits • Climate Change • Indigenous Issues • Gender Equality 7 National Unity Prairies and the West Québéc 8 Federal Fiscal Outlook • Parliamentary Budget Officer’s most recent forecast has downgraded predicted growth for the economy • The Liberal platform costing projected adding $31.5 billion in new debt over the next four years 9 The Conservatives • Campaigned on cutting regulatory burden, review of “corporate welfare” • Mr. Scheer called a special caucus meeting on December 12 where he announced he was stepping -

March 23, 2020 the Honourable Patty Hajdu, PCMP Minister of Health 70
March 23, 2020 The Honourable Patty Hajdu, P.C. M.P. Minister of Health 70 Colombine Driveway Tunney's Pasture Postal Location: 0906C Ottawa, Ontario K1A 0K9 Subject: COVID-19 and request to pause federal PMPRB regulations Dear Minister Hajdu, We are writing to urgently request, in light of the COVID-19 pandemic health crisis, that you and your Cabinet colleagues pause implementation of changes to the price control regulations of the Patented Medicines Prices Review Board (PMPRB) set to come into force on July 1, 2020. We are signing this letter on behalf of national patient organizations, including health charities and not-for-profits, which are part of Alliance des patients pour la santé, Best Medicines Coalition, Canadian Organization for Rare Disorders, Health Charities Coalition of Canada, and Regroupement québécois des maladies orphelines/Quebec Coalition of Orphan Diseases. Together, we represent millions of Canadian patients with all types of illness, conditions and unmet therapeutic needs. We fully recognize the significant challenges in addressing and mitigating the impact of the COVID-19 crisis to save lives, including communicating national efforts, and we commend you on your role in this vital work. We greatly appreciated the work to ensure the flow of live-saving medicines given the partial closure of the USA-Canada border. We fully support Prime Minister Justin Trudeau’s statement today that it is time for all hands on deck. This effort must involve national leaders along with all stakeholders, including patient organizations that are a vital link and support to patients and indeed all Canadians. The COVID-19 crisis requires full, sustained and undivided attention. -

Environmental
Back to normal is still a long way off Gwynne Dyer p. 12 What now of the Michael environmental Harris movement in Canada? p.11 Phil Gurski p. 11 Some MPs donating their salary increases to charities p. 4 THIRTY-FIRST YEAR, NO. 1718 CANADA’S POLITICS AND GOVERNMENT NEWSPAPER MONDAY, APRIL 13, 2020 $5.00 News Remote caucus meetings Analysis Feds’ response Analysis: Did In the time of the pandemic, the feds flip- flop on closing Liberals holding national caucus the border or wearing meetings seven days a week masks amid The Liberals' daily Liberals meetings start with the COVID-19 are using a an update for MPs on new developments outbreak? regular daily and the government's initiatives from BY PETER MAZEREEUW conference Deputy House Leader Kirsty Duncan, he federal government says call for their left, International science and expert advice is Trade Minister Mary T caucus behind its decision to shut the Ng, and Minister border to travellers and its chang- meetings. The of Middle Class ing advice on whether Canadians Prosperity Mona should wear masks amid the CO- Conservatives Fortier. Usually, VID-19 outbreak. While Canada’s a member of the are using top health official pointed to COVID-19 cabinet new science related to using face Zoom and committee, or masks, one expert says there is another cabinet no scientific evidence that could the New minister also joins have informed Canada’s decision them in updating Democrats to close its border on March 16. caucus members. “There is no science about The Hill Times are using whether it works to restrict all photographs by travel into a country,” said Steven GoToMeeting. -

April 21, 2016 the Honourable Jean-Yves Duclos Minister For
April 21, 2016 The Honourable Jean-Yves Duclos Minister for Families, Children and Social Development House of Commons Ottawa, ON, K1A 0A6 Dear Minister, I am writing to you today on behalf of the Canadian Network of Women’s Shelters and its 13 provincial and territorial shelters associations. The Network provides a unified voice on the issue of violence against women and brings forward the issues and concerns of the shelter sector. As a sector, we are encouraged by your government’s recent announcement to commit 89.9 million dollars over the next two years for shelter construction and renovation. We do, however have several questions and concerns and do feel that it was a lost opportunity that our Network was not consulted following this announcement. We are well positioned to provide advice and expertise in terms of how to ensure that these funds maximize their potential outcomes. This being said, it is not too late and we hope we will have the opportunity to engage in meaningful dialogue in the very near future. As a Network working at the pan Canadian level we appreciate the challenges and the rewards of collaboration. The Budget indicates that the funds will be provided under the Affordable Housing Initiative (AHI) and that provinces and territories will not be required to cost-match these investments. The following questions are, however, not addressed: . How will the funds be rolled out - though a federal mechanism or via the provinces and territories? . How will allocations be made, what is the funding formula to be used? . Will the funds cover infrastructure costs associated to Second Stage Shelters and Shelter outreach programs? In figures provided by your Department, we noticed that under the IAH initiative, Federal contributions to create or renovate shelter beds in Nunavut between 2011-12 and 2014-15 totaled a mere $40,000. -
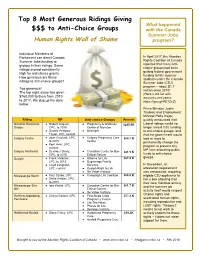
Top 8 Most Generous Ridings Giving $$$ to Anti-Choice Groups
Top 8 Most Generous Ridings Giving What happened $$$ to Anti-Choice Groups with the Canada Summer Jobs Human Rights Wall of Shame program? Individual Members of Parliament can direct Canada In April 2017, the Abortion Summer Jobs funding to Rights Coalition of Canada groups in their ridings. Some reported that many anti- ridings scored consistently choice groups had been getting federal government high for anti-choice grants. funding to hire summer How generous are these students under the Canada ridings to anti-choice groups? Summer Jobs (CSJ) program – about $1.7 Too generous! million since 2010! The top eight alone has given (Here’s the list with $760,000 to them from 2010 amounts and years: to 2017. We dug up the data https://goo.gl/4C1ZsC) below. Prime Minister Justin Trudeau and Employment Minister Patty Hajdu Riding MP Anti-choice Groups Amount quickly announced that Moncton-Riverview- ● Robert Goguen, ● Pregnancy & Wellness $285.6K Liberal ridings would no Dieppe CPC, to 2015 Centre of Moncton longer award CSJ funding ● Ginette Petitpas- ● Birthright to anti-choice groups, and Taylor, LPC, current that the government would Calgary Centre ● Joan Crockatt, CPC, ● Calgary Pregnancy Care $84.7 K look at ways to to 2015 Centre permanently change the ● Kent Hehr, LPC, program to prevent any current MP from allocating public Calgary Northeast ● Devinder Shory, ● Canadian Centre for Bio- $81.5 K CPC, to 2015 Ethical Reform funds to anti-choice groups. Guelph ● Frank Valeriote, ● Alliance for Life $67.9 K LPC, to 2015 ● Beginnings Family -

October 9, 2020 Table of Contents
October 9, 2020 Table of Contents Research No consensus on broad COVID-19 study during rst HESA meeting of current session INTERVIEW: Health minister Hajdu currently ‘not open to delaying’ drug pricing changes Upcoming Events Webinar: “Using Data to Make Public Health Decisions” Press Releases Intergovernmental Aairs Jack.org Federal Economic Development Agency for Southern Ontario Canadian Institutes of Health Research Indigenous Services Canada Statistics Canada The Royal Society of Canada October 9, 2020 RESEARCH No consensus on broad COVID-19 study during rst HESA meeting of current session RESEARCH EXCLUSIVE | OCTOBER 9, 2020 After two and a half hours of continuous debate on the merits of a broad COVID-19 study proposed by Conservative health critic Michelle Rempel Garner (Calgary Nose Hill, Alta.), the health committee’s rst meeting was adjourned with no decisions made. Rempel Garner proposed the health committee (HESA) undertake a study to look at 17 different elements, including rapid, at-home testing; vaccine development; long-term care protocols within federal jurisdiction; the Public Health Agency of Canada ’s Global Public Health Intelligence Network; contact tracing protocol; and Canada’s level of preparedness for future pandemics. The motion also proposed that various ministers, including those for health, procurement and public safety, be required to appear before the committee separately for three hours each in order to answer questions about the government’s response to COVID-19. Rempel Garner’s motion was introduced after opposition members of the committee voted to adjourn the debate on a motion presented by Liberal MP and committee member Tony Van Bynen (Newmarket-Aurora, Ont.), who was calling for a study on the mental health impacts of COVID-19 on Canadians. -

Acentury Inc. 120 West Beaver Creek Rd., Unit 13 Richmond Hill, Ontario Canada L4B 1L2
Acentury Inc. 120 West Beaver Creek Rd., Unit 13 Richmond Hill, Ontario Canada L4B 1L2 Director General, Telecommunications and Internet Policy Branch Innovation, Science and Economic Development Canada 235 Queen Street, 10th Floor Ottawa, Ontario K1A 0H5 February 13, 2020 Subject: Petition to the Governor in Council to Vary Telecom Order CRTC 2019-288, Follow-up to Telecom Orders 2016-396 and 2016-448 – Final rates for aggregated wholesale high-speed access services, Reference: Canadian Gazette, Part 1, August 2019, (TIPB-002-2019) Dear Director General, Telecommunications and Internet Policy Branch, Innovation, Science and Economic Development Canada: I’m writing this letter in response to the CRTC decision on August 2019 under section 12 of the Telecommunications Act issued by the Canadian Radio-television and Telecommunications Commission (CRTC) concerning final rates for aggregated wholesale high-speed access services. As a valued supplier for all the major Canadian Telecommunication companies, I felt obliged to communicate the impact this decision will have on a growing Canadian technology company like ourselves. Acentury is an aspiring technology company who is currently one of the top 500 Canadian growing businesses as reported by Canadian Business (2019) and also one of the top 400 Canadian growing companies as reported by the Globe and Mail (2019). Our achievement and continued success are a direct result of the investment commitment made to next generation 5G and IoT wireless communications led by Bell Canada, Rogers and Telus. Canadian suppliers like us have been supported by Canadian Tier 1 telcos to help build and innovate our technical core competencies and capabilities; it has helped cultivate the growth of a Canadian-led, global organization that can keep pace and compete with our global technology peers. -

Lobbyists, Non-Profits Clamour to Be Heard P. 6
Coming out on the other side of COVID-19 Lobbyists, Wage subsidies critical for post- non-profi ts coronavirus clamour to survival, say be heard p. 6 businesses p. 3 THIRTY-FIRST YEAR, NO. 1715 CANADA’S POLITICS AND GOVERNMENT NEWSPAPER WEDNESDAY, APRIL 1, 2020 $5.00 News Health care Mental health a ‘missing piece’ in feds’ COVID-19 response, say critics, advocates NDP MP Don Davies says the situation calls for an ‘extraordinary response’ and supports for mental health, which one advocate says should come ‘imminently.’ Health Minister Patty BY SAMANTHA WRIGHT ALLEN At least one organization said Hajdu told Senators they expect an announcement on March 25 that the s the government rapidly “imminently,” though neither federal government was Aresponds to the immediate Health Canada nor Health Minis- working on a virtual health and economic needs of ter Patty Hajdu (Thunder Bay- mental health tool Canadians affected by CO- Superior North, Ont.) responded as part of Canada's VID-19, more than two weeks to requests to confi rm the details. COVID-19 response, after the country adopted strict On March 25, Ms. Hajdu said which one group says isolation measures, advocates the government would launch a should be announced say now is the time to address free virtual mental health tool in 'imminently.' The Hill the mental health toll more are Times photograph by Andrew Meade likely to face. Continued on page 16 News Trade News COVID-19 Food supply, emergency vehicle repair: Introduction of electronic, remote voting not Publications Mail Agreement #40068926 keeping Canada-U.S. -

1 Implications of Shareholder Activism
Implications of Shareholder Activism Anita Anand Abstract This chapter asserts that shareholder democracy, or the ability of shareholders to influence the corporation through their vote, underpins the legitimacy of shareholder activism. Examining the empirical literature that evidences the benefits of shareholder activism, the essay argues in favour of increased shareholder representation in director nominations by proxy, not only for the sake of shareholder democracy, but also for the overall benefit of the corporation. 1. Introduction Shareholder activism has become an increasingly conspicuous aspect of capital market activity. The term “activist investor” describes an investor, often a hedge fund, which seeks to acquire significant minority positions in undervalued public companies. The investor may thereafter seek value-enhancing changes in the leadership, governance, capital structure or strategy and operations of the corporation. Such activism engages several aspects of corporate and securities law. Is the relevant law in need of amendment? In answering the question, a starting point is Berle and Means’ (1933, 6) argument that the modern corporation is one characterized by the separation of ownership and control given that “the position of ownership has changed from that of an active to that of a passive agent.”1 The rise of activist shareholders over the past 25 years undermines Berle and Means’ conclusion: these shareholders are anything but passive.2 They are sophisticated, often seeking governance changes over and above those that yield a mere return on their investment. 1 Gilson and Gordon (2013, 863) argue that the rise of shareholder activism has led to a ‘reconcentration of ownership in the hands of institutional investment intermediaries.’ Yet this reconcentration does not capture the full gamut of activist pressure. -

Debates of the House of Commons
43rd PARLIAMENT, 1st SESSION House of Commons Debates Official Report (Hansard) Volume 149 No. 032 Tuesday, March 24, 2020 Speaker: The Honourable Anthony Rota CONTENTS (Table of Contents appears at back of this issue.) 2067 HOUSE OF COMMONS Tuesday, March 24, 2020 The House met at 12 p.m. [English] I wish to inform the House that pursuant to Standing Order 28(3), the Speaker sent a notice calling the House to meet this day, Prayer and I now lay this on the table. [Translation] ● (1200) What is more, on Sunday, March 22, the Speaker sent every [English] member a message explaining why the House was being recalled. I would also like to inform the House that as part of the steps taken RECALL OF THE HOUSE OF COMMONS by the government under Standing Order 55(1), the Speaker pub‐ The Deputy Speaker: Colleagues, before we begin our proceed‐ lished a special Order Paper and Notice Paper giving notice of a ings, I would like to say a few words. government bill. [English] We all recognize that this is a highly unusual sitting, given the extraordinary circumstances in which we all find ourselves present‐ I also wish to lay upon the table a letter from the Leader of the ly. Government in the House of Commons, dated March 22, 2020. [Translation] [Translation] I recognize the Leader of the Government in the House of Com‐ As a result, you will notice that the arrangements we are used to mons. are different today. We are fewer in number and other special mea‐ sures have been put in place based on the recommendations of pub‐ [English] lic health officials. -

Joint Letter to Minister Duclos on Basic Income Pilot
September 10th, 2018 The Honourable Jean-Yves Duclos Minister of Families, Children & Social Development House of Commons Ottawa, ON K1A 0A6 Dear Minister Duclos: On September 4th, the Mayors of Hamilton, Brantford, Kawartha Lakes, and Thunder Bay wrote a joint letter to you, asking the Federal Government to assume oversight of the Ontario Basic Income Pilot project. The Hamilton Chamber of Commerce and Thunder Bay Chamber of Commerce strongly encourage the Federal Government to consider this request. The economy has and will continue to drastically change in the years ahead. With developing technologies and increased automation, government policy needs to adapt in order to respond to potential disruptions in the labour market. If the Ontario Basic Income Pilot project had reached its planned conclusion, it would have provided lawmakers the data they need to evaluate the merit of this approach to managing market disruption. The basic income project was intended to help guide evidence-based policy-making. That is why we, in conjunction with the Ontario Chamber of Commerce, were supportive of the pilot and interested in its outcome. Federal oversight of the pilot would revive the critical information generated over the full course of the experiment. In particular, we are keen to understand if basic income payments: ● Influence recipients’ socio-economic outcomes, their participation in the labour market, and/or their uptake of education/training opportunities; ● Alter participants’ use of existing social and/or income redistribution programs; ● Lead to an increase in entrepreneurial activity. As of March 2019, those participating in Ontario Basic Income Pilot project will receive their last payment from the Provincial Government. -

Cabinet Committee Mandate and Membership
Cabinet Committee Mandate and Membership Current as of September 28, 2020 The Deputy Prime Minister and Minister of Finance and the Minister of Middle Class Prosperity and Associate Minister of Finance are ex-officio members of Committees where they are not shown as standing members. The Honourable James Gordon Carr, P.C. will be invited to attend committee meetings at the request of Committee Chairs. Cabinet Committee on Agenda, Results and Communications Addresses major issues affecting national unity and the strategic agenda of the government, tracks progress on the government’s priorities, coordinates the implementation of the government’s overall agenda, and considers strategic communications issues. Chair: The Rt. Hon. Justin P. J. Trudeau Vice-Chair: The Hon. Chrystia Freeland Members The Hon. Navdeep Singh Bains The Hon. James Gordon Carr The Hon. Mélanie Joly The Hon. Dominic LeBlanc The Hon. Carla Qualtrough The Hon. Pablo Rodriguez The Honourable James Gordon Carr, the Special Representative for the Prairies, will be invited to attend meetings. Treasury Board Acts as the government’s management board. Provides oversight of the government’s financial management and spending, as well as oversight on human resources issues. Provides oversight on complex horizontal issues such as defence procurement and modernizing the pay system. Responsible for reporting to Parliament. Is the employer for the public service, and establishes policies and common standards for administrative, personnel, financial, and organizational practices across government. Fulfills the role of the Committee of Council in approving regulatory policies and regulations, and most orders-in-council. Chair: The Hon. Jean-Yves Duclos Vice-Chair: The Hon.