Paul Ryan on Fox News Sunday Transcript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Talk Shows Between Obama and Trump Administrations by Jack Norcross — 69
Analysis of Talk Shows Between Obama and Trump Administrations by Jack Norcross — 69 An Analysis of the Political Affiliations and Professions of Sunday Talk Show Guests Between the Obama and Trump Administrations Jack Norcross Journalism Elon University Submitted in partial fulfillment of the requirements in an undergraduate senior capstone course in communications Abstract The Sunday morning talk shows have long been a platform for high-quality journalism and analysis of the week’s top political headlines. This research will compare guests between the first two years of Barack Obama’s presidency and the first two years of Donald Trump’s presidency. A quantitative content analysis of television transcripts was used to identify changes in both the political affiliations and profession of the guests who appeared on NBC’s “Meet the Press,” CBS’s “Face the Nation,” ABC’s “This Week” and “Fox News Sunday” between the two administrations. Findings indicated that the dominant political viewpoint of guests differed by show during the Obama administration, while all shows hosted more Republicans than Democrats during the Trump administration. Furthermore, U.S. Senators and TV/Radio journalists were cumulatively the most frequent guests on the programs. I. Introduction Sunday morning political talk shows have been around since 1947, when NBC’s “Meet the Press” brought on politicians and newsmakers to be questioned by members of the press. The show’s format would evolve over the next 70 years, and give rise to fellow Sunday morning competitors including ABC’s “This Week,” CBS’s “Face the Nation” and “Fox News Sunday.” Since the mid-twentieth century, the overall media landscape significantly changed with the rise of cable news, social media and the consumption of online content. -
Saturday Listings Sunday Listings
6 – THURSDAY, SEPTEMBER 10, 2020 GAINESVILLE DAILY REGISTER SATURDAY LISTINGS SEPTEMBER 12 PRIME TIME S1 – DISH NETWORK S2 - DIRECTV 6 PM 6:30 7 PM 7:30 8 PM 8:30 9 PM 9:30 10 PM 10:30 11 PM S1S2 Fox 4 News Saturday (N) MLB Baseball Houston Astros at Los Angeles Dodgers Site: Dodger Stadium -- Los Angeles, Calif. (L) Fox 4 News at 9:00 p.m. (N) Labor of Love (4) "10 Things Kristy 4 4 KDFW (4) Likes About You" NBC 5 News at Wingstop NHL Hockey Stanley Cup Playoffs (L) NBC5 News at Saturday Night Live (5) 6:00 p.m. Inside High 10:00 p.m. (N) 5 5 KXAS (5) Saturday (N) School Sports Jeopardy! Wheel Fortune FC Dallas Live MLS Soccer FC Dallas at Houston Dynamo Site: BBVA Compass NCIS: New Orleans "Powder Madam Secretary "So It Goes" (21) "Delicious (L) Stadium -- Houston, Texas (L) Keg" 21 21 KTXA (6) Destinations" Religious Programming Michael Kenneth W. The Green In Touch With Dr. Charles Perry Stone: Love Israel Israel: The (2) Youssef Hagin Room Stanley Manna-Fest With Baruch Prophetic 2 KDTN (7) Korman Connection College Football College Football Scoreboard (L) /NCAA Football Clemson at Wake Forest Site: BB&T Field -- College News 8 Entertainment Tonight (8) Scoreboard (L) Winston-Salem, N.C. (L) Football Update at Weekend 8 8 WFAA (8) Scoreboard (L) 10:00 p.m. (N) <++++ Titanic (1997, Drama) Leonardo DiCaprio, Victor Garber, Kate Winslet. Noticiero Noticias Somos cowboys (39) Telemundo 39 Telemundo Fin 39 39 KXTX (9) at 10pm (N) de semana (N) CBS 11 News at Paid Program CBS Saturday Encore Love Island: More to Love (N) 48 Hours (SP) (N) CBS 11 News Saturday at Cowboys (11) 6:00 p.m. -

Clear Channel Radio Names Fox News As Primary National News Provider to More Than 100 News/Talk Stations
Contact: Omar Thompson Irena Briganti Clear Channel Radio Fox News (210) 822-2828 (212) 301-3608 [email protected] [email protected] Kim Holt/Jen Gery Brainerd Communicators, Inc. (212) 986-6667 [email protected] [email protected] Clear Channel Radio Names Fox News as Primary National News Provider to More Than 100 News/Talk Stations Service Includes Five-Minute Top-of-the-Hour Newscast, Nightly Signature Newscast and Dedicated 24/7 National News Coverage Deal Creates Direct Competitor to ABC Radio and CBS Radio San Antonio and New York – December 6, 2004 – Clear Channel Radio and Fox News today announced that Fox News Radio will be the primary national news service for more than 100 of Clear Channel’s news/talk stations under a five-year deal beginning in 2005. The agreement incorporates many of Clear Channel Radio’s most prominent news/talk stations, including those in Los Angeles, San Diego, Phoenix and Atlanta. Fox’s new full-service, general-interest news offering will feature Fox News’ top talent and include a five-minute top-of-the-hour newscast, a nightly signature news broadcast, and 24/7 dedicated national news coverage. Fox will also be the primary provider for breaking national news. Also under the agreement, Fox News Radio will have access to news produced by Clear Channel’s more than 500 local news journalists via the company’s Clear Channel News Network. “Working this closely with a premier national news provider for the majority of our news/talk stations makes overwhelming sense,” said John Hogan, chief executive officer of Clear Channel Radio. -

Fox Corporation Annual Report 2020
Fox Corporation Annual Report 2020 Form 10-K (NASDAQ:FOXA) Published: August 10th, 2020 PDF generated by stocklight.com UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended June 30, 2020 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 001-38776 FOX CORPORATION (Exact Name of Registrant as Specified in its Charter) Delaware 83-1825597 (State or Other Jurisdiction of (I.R.S. Employer Incorporation or Organization) Identification No.) 1211 Avenue of the Americas, New York, New York 10036 (Address of Principal Executive Offices) (Zip Code) Registrant’s telephone number, including area code (212) 852-7000 Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbols Name of Each Exchange on Which Registered Class A Common Stock, par value $0.01 per share FOXA The Nasdaq Global Select Market Class B Common Stock, par value $0.01 per share FOX The Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. -
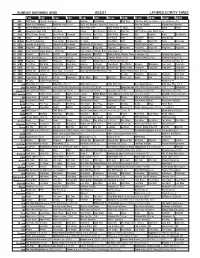
Sunday Morning Grid 8/22/21 Latimes.Com/Tv Times
SUNDAY MORNING GRID 8/22/21 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Face the Nation (N) News Graham Bull Riding PGA Tour PGA Tour Golf The Northern Trust, Final Round. (N) 4 NBC Today in LA Weekend Meet the Press (N) Å 2021 AIG Women’s Open Final Round. (N) Race and Sports Mecum Auto Auctions 5 CW KTLA 5 Morning News at 7 (N) Å KTLA News at 9 KTLA 5 News at 10am In Touch David Smile 7 ABC Eyewitness News 7AM This Week Ocean Sea Rescue Hearts of Free Ent. 2021 Little League World Series 9 KCAL KCAL 9 News Sunday Joel Osteen Jeremiah Joel Osteen Paid Prog. Mike Webb Harvest AAA Danette Icons The World’s 1 1 FOX Mercy Jack Hibbs Fox News Sunday The Issue News Sex Abuse PiYo Accident? Home Drag Racing 1 3 MyNet Bel Air Presbyterian Fred Jordan Freethought In Touch Jack Hibbs AAA NeuroQ Grow Hair News The Issue 1 8 KSCI Fashion for Real Life MacKenzie-Childs Home MacKenzie-Childs Home Quantum Vacuum Å COVID Delta Safety: Isomers Skincare Å 2 2 KWHY Programa Resultados Revitaliza Programa Programa Programa Programa Programa Programa Programa Programa Programa 2 4 KVCR Great Scenic Railway Journeys: 150 Years Suze Orman’s Ultimate Retirement Guide (TVG) Great Performances (TVG) Å 2 8 KCET Darwin’s Cat in the SciGirls Odd Squad Cyberchase Biz Kid$ Build a Better Memory Through Science (TVG) Country Pop Legends 3 0 ION NCIS: New Orleans Å NCIS: New Orleans Å Criminal Minds (TV14) Criminal Minds (TV14) Criminal Minds (TV14) Criminal Minds (TV14) 3 4 KMEX Programa MagBlue Programa Programa Fútbol Fútbol Mexicano Primera División (N) República deportiva (N) 4 0 KTBN R. -

Audio and Podcasting Fact Sheet
NUMBERS, FACTS AND TRENDS SHAPING YOUR WORLD ABOUT FOLLOW MY ACCOUNT DONATE Journalism & Media ARCH MENU RESEARCH AREAS FACT HT JUN 16, 2017 Audio and Podcasting Fact Sheet MOR FACT HT: TAT OF TH NW MDIA Audience conomic The audio news sector in the U.S. is split by modes of delivery: traditional terrestrial (AM/FM) radio and digital formats such as online radio and podcasting. While terrestrial radio reaches almost the entire U.S. population and Ownerhip remains steady in its revenue, online radio and podcasting audiences have continued to grow over the last decade. Explore the patterns and longitudinal data about audio and podcasting below. Data on public radio is available in a Find out more separate fact sheet. Audience The audience for terrestrial radio remains steady and high: In 2016, 91% of Americans ages 12 or older listened to terrestrial radio in a given week, according to Nielsen Media Research data published by the Radio Advertising Bureau, a figure that has changed little since 2009. (Note: This and most data on the radio sector apply to all types of listening and do not break out news, except where noted.) Weekl terretrial radio litenerhip Chart Data hare med % of Americans ages 12 or older who listen to terrestrial (AM/FM) radio in a given week Year % of American age 12 or older who liten to terretrial (AM/FM) radio in a given week 2009 92% 2010 92% 2011 93% 2012 92% 2013 92% 2014 91% 2015 91% 2016 91% ource: Nielen Audio RADAR 131, Decemer 2016, pulicl availale via Radio Advertiing ureau. -

Audio and Podcasting Fact Sheet
NUMBERS, FACTS AND TRENDS SHAPING YOUR WORLD ABOUT FOLLOW MY ACCOUNT DONATE Journalism & Media SEARCH MENU RESEARCH AREAS FACT SHEET JULY 12, 2018 Audio and Podcasting Fact Sheet MORE FACT SHEETS: STATE OF THE NEWS MEDIA Audience Economics The audio news sector in the United States is split by modes of delivery: traditional terrestrial (AM/FM) radio and digital formats such as online radio and podcasting. While terrestrial radio reaches almost the entire U.S. population Ownership and remains steady in its revenue, online radio and podcasting audiences have continued to grow over the past decade. Explore the patterns and longitudinal data about audio and podcasting below. Data on other public radio Find out more beyond podcasting are available in a separate fact sheet. Audience The audience for terrestrial radio remains steady and high: In 2017, 90% of Americans ages 12 and older listened to terrestrial radio in a given week, according to Nielsen Media Research data published by the Radio Advertising Bureau, a figure that has changed little since 2009. Note: This and most data on the radio sector apply to all types of listening and do not break out news, except where noted. Nielsen lists news/talk among the most listened-to radio formats; in 2017, the news/talk format earned 9.9% of radio audiences during any 15-minute period during the day. Weekly terrestrial radio listenership Chart Data Share Embed % of Americans ages 12 and older who listen to terrestrial (AM/FM) radio in a given week % of Americans ages 12 and older Year who listen to terrestrial (AM/FM) radio in a given week 2009 92% 2010 92% 2011 93% 2012 92% 2013 92% 2014 91% 2015 91% 2016 91% 2017 90% Source: Nielsen Audio RADAR 136, March 2018, publicly available via Radio Advertising Bureau. -
A Chance to GO NUTS FDA Panel Recommends Approval for New Peanut Allergy Drug
The Gazette | Sunday, September 29, 2019 | Section C Your contact: Sections Editor Shari Rampenthal, 608-755-8394, [email protected] A chance to GO NUTS FDA panel recommends approval for new peanut allergy drug By Lisa Schencker He gave her a tiny bit of peanut pro- recommended approval of the tein and monitored her in his office for first drug designed to Chicago Tribune allergic reactions. Gradually, he stepped reduce allergic reactions up the amount she ate, over the course in children with pea- CHICAGO of about 10 months. Today, peanut but- nut allergies. The rec- Lauren Tilmont didn’t believe ter still upsets her stomach, but she can ommendation makes it when her doctor told her a few snack on Snickers bars and munch on it likely that the drug, years ago that he had a treat- peanut M&Ms without a problem. Palforzia, made by Aim- Tilmont called the treatment the mune Therapeutics, will ment that might allow her to most difficult thing she’s ever done, but get FDA approval. eat peanuts, despite a lifelong she no longer fears accidentally touch- The drug is not meant as a cure or a allergy to them. ing a surface that has peanuts on it or path to snacking on peanuts. Rather, it’s “The first thing I told him was, eating at restaurants that use peanut oil. designed to decrease the amount and “It has empowered me,” she said. severity of allergic reactions after acci- ‘You’re crazy. That doesn’t hap- It’s a somewhat controversial treat- pen,’ ” said Tilmont, 25, of Rog- dental exposure to peanuts. -

TWENTY-FIRST CENTURY FOX, INC. (Exact Name of Registrant As Specified in Its Charter)
21st Century Fox - Annual Report Page 1 of 201 ^ \ ] _ UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended June 30, 2017 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 001-32352 TWENTY-FIRST CENTURY FOX, INC. (Exact Name of Registrant as Specified in its Charter) Delaware 26-0075658 (State or Other Jurisdiction of (I.R.S. Employer Incorporation or Organization) Identification No.) 1211 Avenue of the Americas, New York, New York 10036 (Address of Principal Executive Offices) (Zip Code) Registrant’s telephone number, including area code (212) 852-7000 Securities registered pursuant to Section 12(b) of the Act: Name of Each Exchange On Which Title of Each Class Registered Class A Common Stock, par value $0.01 per share The NASDAQ Global Select Market Class B Common Stock, par value $0.01 per share The NASDAQ Global Select Market Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act of 1933. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934. -

Left in the Dark (Pdf)
LEFT IN THE DARK Local Election Coverage in the Age of Big-Money Politics By Timothy Karr Free Press September 2012 www.freepress.net 1 Executive Summary With more than $3.3 billion in political ad spending projected by Election Day, Free Press has turned its attention to the local television stations airing these ads. Left in the Dark explores whether stations barraging viewers with political ads are balancing this out with coverage of the role money is playing in this year’s elections. We focus on stations in five cities where ad spending has skyrocketed this year: Charlotte, Cleveland, Las Vegas, Milwaukee and Tampa. Left in the Dark asks the following questions: • Are these stations reporting on the Super PACs and other “nonaligned” groups behind so many of the political ads airing in these cities? • Are these stations reporting on the role television stations and their parent companies play as recipients of political ad money? • Are these stations fact-checking political ads airing in their markets? First, Left in the Dark analyzes coverage on Milwaukee’s ABC, CBS, Fox and NBC affiliates in the two weeks prior to Wisconsin’s June 5 recall election. This period saw an increase in ad spending similar to what stations in other battleground markets will expect before November’s general election. Free Press sent volunteers into Milwaukee stations, where they inspected and photocopied broadcasters’ political files to identify the groups most actively buying political ads before the recall. We checked for mentions of these groups and their political ads in local news coverage. -

Wlax/Weux Program Schedule
WLAX/WEUX PROGRAM SCHEDULE Monday Tuesday Wednesday Thursday Friday Saturday Sunday 06/11/2018 06/12/2018 06/13/2018 06/14/2018 06/15/2018 06/16/2018 06/17/2018 How I Met Your Mother I How I Met Your Mother I How I Met Your Mother I Judge Judy I Judge Judy I Family Feud Judge Judy 4:00 am epi#: 7ALH21 epi#: 7ALH24 epi#: 8ALH01 epi#: 5505 epi#: 5287 epi#: 17126 epi#: 4409 Century Premiere Century Premiere Century Premiere HD TV-G WKNDM1 WKNDM1 TV-14 TV-14 TV-14 TV-G TV-PG TV-G How I Met Your Mother II How I Met Your Mother II How I Met Your Mother II Judge Judy II Judge Judy II Family Feud Judge Judy 4:30 am epi#: 5ALH03 epi#: 5ALH04 epi#: 5ALH05 epi#: 5325 epi#: 5357 epi#: 16101 epi#: 4408 Century Premiere Century Premiere Century Premiere TV-G TV-G WKNDM2 WKNDM2 TV-PG TV-PG TV-PG TV-PG TV-G Paid Program Shepherd's Chapel Shepherd's Chapel Shepherd's Chapel Shepherd's Chapel Shepherd's Chapel U.S. Farm Report 5:00 am TV-G TV-G TV-G TV-G TV-G epi#: 2340 TV-PG 5:30 am Paid Program Paid Program Paid Program Paid Program Hot Bench I Hot Bench I Person of Interest FIFA World Cup LIVE 6:00 am epi#: 1768 epi#: 1710 epi#: 92 FOX - LIVE HD HD WKNDM1 TV-G TV-G TV-14 Paid Program Paid Program Paid Program Hot Bench Hot Bench II 6:30 am epi#: 1682 epi#: 1727 HD HD TV-G TV-G The Jason Show The Jason Show The Jason Show Maury Veggie Tales I FIFA World Cup Today FIFA World Cup Costa Rica Vs. -

Twenty-First Century Fox, Inc. 2016 Annual Report
2016 ANNUAL REPORT The Last Man On Earth Fox Broadcasting Company We foster a culture of originality and innovation to make 21st Century Fox a place where fresh ideas, new approaches and compelling stories can take root. We are bold because we believe audiences always want, and deserve, more choice and better experiences. Our core video brands, and the breakout content that powers them, are indispensable for customers and distributors around the globe. 21st Century Fox is well positioned to build on our leadership and continue driving innovation for customers the world over. 21st Century Fox Annual Report Chairmen’s Letter 2016 Dear Fellow Stockholders 21st Century Fox Annual Report Chairmen’s Letter Our strategic plan is built around three fundamental As this development unfolds, the question is: where priorities: delivering standout creative output to power will value accrue? The answer is revealing itself. our core brands FOX, National Geographic, Fox News, Companies like ours that are upstream, investing at FOX Sports, FX and STAR India; driving innovation scale in producing compelling content that consumers for customers across multiple platforms; and further love and demand, building and supporting brands that advancing our capabilities to monetize our content have meaning and resonance, and nurturing cultures wherever it is consumed. This past Fiscal Year we made of creative risk-taking are best positioned to succeed. solid progress on all three fronts and delivered growth We are seeing the evidence to support this conviction in adjusted revenues and EBITDA for the year. every day. We are experiencing growth in both our Our major accomplishments against our key strategic affiliate fee revenue and advertising revenue.