(In Particular Avian Influenza) from Wild Aquatic Birds in North Queensland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bird Notes Quarterly Newsletter of the Western Australian Branch of Birdlife Australia No
Western Australian Bird Notes Quarterly Newsletter of the Western Australian Branch of BirdLife Australia No. 173 March 2020 birds are in our nature Members in the field World Wetlands Day bird walk Albany, p 32, photo by White-winged Fairy-wren at Mullaloo, photo by Caroline Shaun Welsh Mynott So hot for Brown-headed Honeyeater, p 42, photo Delene Osprey with catch, photo by Garry Taylor van Dyk David Budd rescuing two Eurasian Coots at Mandurah, p34 Bibra Walk, p 36, photo by Alan Watson Front cover: Red-necked Stint at Rottnest Island - photo by Clive Nealon Page 2 Western Australian Bird Notes, No. 173 March 2020 Western Australian Branch of EXECUTIVE COMMITTEE, 2020 BirdLife Australia Chair: Mr Viv Read Office: Peregrine House 167 Perry Lakes Drive, Floreat WA 6014 Vice Chair: Dr Mike Bamford Hours: Monday-Friday 9:30 am to 12.30 pm Secretary: Lou Scampoli Telephone: (08) 9383 7749 E-mail: [email protected] Treasurer: Beverly Winterton BirdLife WA web page: www.birdlife.org.au/wa Committee: Alasdair Bulloch, Mark Henryon, Andrew Hobbs, Chair: Mr Viv Read Peter Jacoby, Jennifer Sumpton and Beth Walker BirdLife Western Australia is the WA Branch of the national organisation, BirdLife Australia. We are dedicated to creating a brighter future for Australian birds. General meetings: Held at the Bold Park Eco Centre, Perry Lakes Drive, Floreat, commencing 7:30 pm on the 4th Monday of the month (except December) – see ‘Coming events’ for details. Executive meetings: Held at Peregrine House on the 2nd Monday of the month. Communicate any matters for consideration to the Chair. -

Australia Comprehensive Target Species Custom Tour Trip Report
AUSTRALIA COMPREHENSIVE TARGET SPECIES CUSTOM TOUR TRIP REPORT 23 AUGUST – 1 OCTOBER 2019 By Andy Walker The Endangered (BirdLife International) Mallee Emu-wren has a tiny global range, and we had excellent views of a pair while birdwatching in Victoria. www.birdingecotours.com [email protected] 2 | TRIP REPORT Australia, Aug-Oct 2019 Overview This 40-day custom birdwatching tour of Australia commenced in Adelaide, South Australia, on the 23rd of August 2019 and ended in Sydney, New South Wales, on the 1st of October 2019. The tour also visited the states and territories of Victoria, Northern Territory, and Queensland. A pelagic trip was taken off southern South Australia (Port MacDonnell). Unfortunately a planned pelagic trip off southern Queensland (Southport) was canceled due to illness. This custom birding tour route was South Australia (Adelaide to Port MacDonnell) - Victoria (circuit around the western section of the state) - New South Wales (a brief stop for parrots along the state border) -Victoria (remainder of the western circuit back to Melbourne) - Northern Territory (Alice Springs area) - Northern Territory (Darwin to Kakadu and back) - Queensland (circuit out of Brisbane) - New South Wales (circuit out of Sydney). Several areas visited on this tour feature in our Australia set departure tours (e.g. East Coast and Northern Territory tours). A list of target birds was provided for the tour (the clients’ third trip to Australia), and these became the focus of the tour route and birding, though new trip birds encountered were also enjoyed! A total of 421 bird species were seen (plus 5 species heard only), including many client target birds. -

West Papua Expedition
The fabulous Spangled Kookaburra was one of the many highlights (Mark Van Beirs) WEST PAPUA EXPEDITION 22/28 OCTOBER – 10 NOVEMBER 2019 LEADER: MARK VAN BEIRS 1 BirdQuest Tour Report: West Papua Expedition www.birdquest-tours.com The cracking Kofiau Paradise Kingfisher posed ever so well (Mark Van Beirs) This unusual trip was set up to fill in some of the remaining gaps in the Birdquest New Guinea lifelist, so the plan was to visit several hard to reach venues in West Papua. The pre-trip was aiming to climb to the top of 2 BirdQuest Tour Report: West Papua Expedition www.birdquest-tours.com Mount Trikora in the Snow Mountains, but because of recent rioting and civil unrest (whereby several dozen people had been killed), access to the town of Wamena was totally denied to foreign visitors by the authorities. So, sadly, no Snow Mountain Robin… We did manage to visit the famous Wasur National Park, which produced the fantastic Spangled Kookaburra and Grey-crowned and Black Mannikins (all Birdquest lifers) and we reached the island of Kofiau, where the fabulous Kofiau Paradise Kingfisher and the modestly- plumaged Kofiau Monarch (two more Birdquest lifers) showed extremely well. The fabulous lowland rainforest site of Malagufuk gave us a long list of exquisite species amongst which a truly impressive Northern Cassowary, a cute Wallace’s Owlet-nightjar, a sublime Papuan Hawk-Owl and a tremendous Red- breasted Paradise Kingfisher stood out. Kingfishers especially performed extremely well on this tour as we saw no fewer than 15 species, including marvels like Hook-billed, Common Paradise, Blue-black, Beach, Yellow-billed and Papuan Dwarf Kingfishers and Blue-winged and Rufous-bellied Kookaburras. -
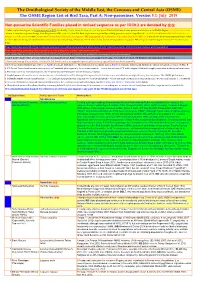
ORL 5.1 Non-Passerines Final Draft01a.Xlsx
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part A: Non-passerines. Version 5.1: July 2019 Non-passerine Scientific Families placed in revised sequence as per IOC9.2 are denoted by ֍֍ A fuller explanation is given in Explanation of the ORL, but briefly, Bright green shading of a row (eg Syrian Ostrich) indicates former presence of a taxon in the OSME Region. Light gold shading in column A indicates sequence change from the previous ORL issue. For taxa that have unproven and probably unlikely presence, see the Hypothetical List. Red font indicates added information since the previous ORL version or the Conservation Threat Status (Critically Endangered = CE, Endangered = E, Vulnerable = V and Data Deficient = DD only). Not all synonyms have been examined. Serial numbers (SN) are merely an administrative convenience and may change. Please do not cite them in any formal correspondence or papers. NB: Compass cardinals (eg N = north, SE = southeast) are used. Rows shaded thus and with yellow text denote summaries of problem taxon groups in which some closely-related taxa may be of indeterminate status or are being studied. Rows shaded thus and with yellow text indicate recent or data-driven major conservation concerns. Rows shaded thus and with white text contain additional explanatory information on problem taxon groups as and when necessary. English names shaded thus are taxa on BirdLife Tracking Database, http://seabirdtracking.org/mapper/index.php. Nos tracked are small. NB BirdLife still lump many seabird taxa. A broad dark orange line, as below, indicates the last taxon in a new or suggested species split, or where sspp are best considered separately. -

Download the Bird List
Bird list for PAIWALLA WETLANDS -35.03468 °N 139.37202 °E 35°02’05” S 139°22’19” E 54 351500 6121900 or new birdssa.asn.au ……………. …………….. …………… …………….. … …......... ……… Observers: ………………………………………………………………….. Phone: (H) ……………………………… (M) ………………………………… ..………………………………………………………………………………. Email: …………..…………………………………………………… Date: ……..…………………………. Start Time: ……………………… End Time: ……………………… Codes (leave blank for Present) D = Dead H = Heard O = Overhead B = Breeding B1 = Mating B2 = Nest Building B3 = Nest with eggs B4 = Nest with chicks B5 = Dependent fledglings B6 = Bird on nest NON-PASSERINES S S A W Code No. NON-PASSERINES S S A W Code No. NON-PASSERINES S S A W Code No. Red-necked Avocet Black Falcon Spur-winged Plover (Masked Lapwing) Rainbow Bee-eater Brown Falcon Australasian Bittern Peregrine Falcon Australian Pratincole Black-backed Bittern Galah Brown Quail Eastern Bluebonnet Black-tailed Godwit Stubble Quail Australian Boobook Cape Barren Goose Buff-banded Rail Brush Bronzewing Brown Goshawk Lewin's Rail Common Bronzewing Australasian Grebe Mallee Ringneck (Australian Ringneck) Budgerigar Great Crested Grebe Cockatiel Hoary-headed Grebe Adelaide Rosella (Crimson Rosella) Sulphur-crested Cockatoo Common Greenshank Eurasian Coot Silver Gull Common Sandpiper Little Corella Hardhead Curlew Sandpiper Great Cormorant Spotted Harrier Marsh Sandpiper Little Black Cormorant Swamp Harrier Pectoral Sandpiper Little Pied Cormorant Nankeen Night Heron Sharp-tailed Sandpiper Pied Cormorant White-faced Heron Wood Sandpiper Australian Crake White-necked -
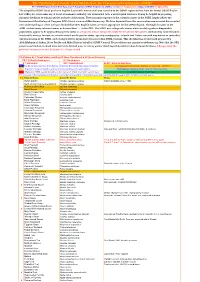
Simplified-ORL-2019-5.1-Final.Pdf
The Ornithological Society of the Middle East, the Caucasus and Central Asia (OSME) The OSME Region List of Bird Taxa, Part F: Simplified OSME Region List (SORL) version 5.1 August 2019. (Aligns with ORL 5.1 July 2019) The simplified OSME list of preferred English & scientific names of all taxa recorded in the OSME region derives from the formal OSME Region List (ORL); see www.osme.org. It is not a taxonomic authority, but is intended to be a useful quick reference. It may be helpful in preparing informal checklists or writing articles on birds of the region. The taxonomic sequence & the scientific names in the SORL largely follow the International Ornithological Congress (IOC) List at www.worldbirdnames.org. We have departed from this source when new research has revealed new understanding or when we have decided that other English names are more appropriate for the OSME Region. The English names in the SORL include many informal names as denoted thus '…' in the ORL. The SORL uses subspecific names where useful; eg where diagnosable populations appear to be approaching species status or are species whose subspecies might be elevated to full species (indicated by round brackets in scientific names); for now, we remain neutral on the precise status - species or subspecies - of such taxa. Future research may amend or contradict our presentation of the SORL; such changes will be incorporated in succeeding SORL versions. This checklist was devised and prepared by AbdulRahman al Sirhan, Steve Preddy and Mike Blair on behalf of OSME Council. Please address any queries to [email protected]. -

Australia Comprehensive Target Species Custom Tour Trip Report
AUSTRALIA COMPREHENSIVE TARGET SPECIES CUSTOM TOUR TRIP REPORT 23 AUGUST – 1 OCTOBER 2019 By Andy Walker The Endangered (BirdLife International) Mallee Emu-wren has a tiny global range, and we had excellent views of a pair while birdwatching in Victoria. www.birdingecotours.com [email protected] 2 | TRIP REPORT Australia, Aug-Oct 2019 Overview This 40-day custom birdwatching tour of Australia commenced in Adelaide, South Australia, on the 23rd of August 2019 and ended in Sydney, New South Wales, on the 1st of October 2019. The tour also visited the states and territories of Victoria, Northern Territory, and Queensland. A pelagic trip was taken off southern South Australia (Port MacDonnell). Unfortunately a planned pelagic trip off southern Queensland (Southport) was canceled due to illness. This custom birding tour route was South Australia (Adelaide to Port MacDonnell) - Victoria (circuit around the western section of the state) - New South Wales (a brief stop for parrots along the state border) -Victoria (remainder of the western circuit back to Melbourne) - Northern Territory (Alice Springs area) - Northern Territory (Darwin to Kakadu and back) - Queensland (circuit out of Brisbane) - New South Wales (circuit out of Sydney). Several areas visited on this tour feature in our Australia set departure tours (e.g. East Coast and Northern Territory tours). A list of target birds was provided for the tour (the clients’ third trip to Australia), and these became the focus of the tour route and birding, though new trip birds encountered were also enjoyed! A total of 421 bird species were seen (plus 5 species heard only), including many client target birds. -

Papua New Guinea Birding in Paradise IV 21St July to 6Th August 2019 (18 Days) Trip Report
Papua New Guinea Birding in Paradise IV 21st July to 6th August 2019 (18 days) Trip Report Flame Bowerbird by Jacques Erard Tour Leaders: Adam Walleyn and Gareth Robbins Trip report compiled by Tour Leader: Adam Walleyn Rockjumper Birding Tours www.rockjumperbirding.com Trip Report – RBL Papua New Guinea – Birding in Paradise IV 2019 2 Tour Summary Our group assembled in Port Moresby and set off on a lovely afternoon for an exploration of the Pacific Adventist University grounds, full of eager anticipation for the epic adventure ahead. A quick stop en route at some rice fields produced several Raja Shelducks and numerous Pied Herons amongst more widespread species. A few hours gentle walking on the grounds yielded all the hoped-for specialties: Black-backed Butcherbird, Fawn-breasted Bowerbird, Grey-headed Mannikin and a pair of massive yet perfectly camouflaged Papuan Frogmouths on their day roost in an enormous raintree. The various water bodies were full of birds including Comb-crested Jacana, Grey Teal, Plumed and Wandering Whistling Ducks, Australian White Ibis, Nankeen Night Heron, Intermediate Egret and many more. Several trees were dripping with fruiting figs Plumed Whistling Duck by Ma Yan Bryant and playing host to unusually large numbers of Orange-fronted and Orange -bellied Fruit Doves, Torresian Imperial Pigeon, Australasian Figbird, Brown Oriole, and Yellow-faced Myna. A very birdy introduction to this amazing country! One of our earliest starts of the tour saw us leaving Port Moresby and winding up the Sogeri Plateau, flushing a Large-tailed Nightjar off the road along the way. In the early morning light, we arrived at the Raggiana Bird-of-paradise lek and could already hear several males giving their raucous calls. -

Fiftee N Vertebrate Beginnings the Chordates
Hickman−Roberts−Larson: 15. Vertebrate Beginnings: Text © The McGraw−Hill Animal Diversity, Third The Chordates Companies, 2002 Edition 15 chapter •••••• fifteen Vertebrate Beginnings The Chordates It’s a Long Way from Amphioxus Along the more southern coasts of North America, half buried in sand on the seafloor,lives a small fishlike translucent animal quietly filtering organic particles from seawater.Inconspicuous, of no commercial value and largely unknown, this creature is nonetheless one of the famous animals of classical zoology.It is amphioxus, an animal that wonderfully exhibits the four distinctive hallmarks of the phylum Chordata—(1) dorsal, tubular nerve cord overlying (2) a supportive notochord, (3) pharyngeal slits for filter feeding, and (4) a postanal tail for propulsion—all wrapped up in one creature with textbook simplicity. Amphioxus is an animal that might have been designed by a zoologist for the classroom. During the nineteenth century,with inter- est in vertebrate ancestry running high, amphioxus was considered by many to resemble closely the direct ancestor of the vertebrates. Its exalted position was later acknowledged by Philip Pope in a poem sung to the tune of “Tipperary.”It ends with the refrain: It’s a long way from amphioxus It’s a long way to us, It’s a long way from amphioxus To the meanest human cuss. Well,it’s good-bye to fins and gill slits And it’s welcome lungs and hair, It’s a long, long way from amphioxus But we all came from there. But amphioxus’place in the sun was not to endure.For one thing,amphioxus lacks one of the most important of vertebrate charac- teristics,a distinct head with special sense organs and the equipment for shifting to an active predatory mode of life. -

Grand Australia Part I: New South Wales & the Northern Territory
GRAND AUSTRALIA PART I: NEW SOUTH WALES & THE NORTHERN TERRITORY SEPTEMBER 20–OCTOBER 6, 2019 Spangled Drongo © Max Breckenridge LEADERS: MAX BRECKENRIDGE & BARRY ZIMMER LIST COMPILED BY: MAX BRECKENRIDGE VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM VENT’s Grand Australia tour has been a staple offering for almost two decades now, led by the insatiable Dion Hobcroft. Dion’s skills as a tour leader and his extensive knowledge of Australia, his home country, meant that the tour has grown immensely in popularity over the years. As such, in 2019, for the first time, VENT offered two subsequent trips for both Part I, Part II, and the extension to Tasmania. I was the primary leader for the first run of Grand Australia, with Dion to follow a week later (and David James in Tasmania). My co-leaders were the two Zimmer brothers, Kevin and Barry, who are two of VENT’s long-serving and most popular tour leaders. Barry joined me for Part I and Kevin was with me for three weeks during Part II and Tasmania. I learned a great deal from both and enjoyed their company immensely, making the whole five weeks of touring very enjoyable. Thanks are definitely in order to both Barry and Kevin, but also to Dion and David for their work in creating these tours. Of course, I must also thank Victor and Barry Lyon for their foresight and for putting their trust in me, as well as the office staff, in particular Erik and Rene, for making it all happen. -

Atherton Tablelands Bird List
BIRD LIST EASTERN GREAT EGRET BROLGA AUSTRALIAN PRATINCOLE PALLID CUCKOO ATHERTON TABLELANDS INTERMEDIATE EGRET RED-NECKED CRAKE SILVER GULL BRUSH CUCKOO Alan’s Wildlife Tours CATTLE EGRET BUFF-BANDED RAIL AUSTRALIAN TERN CHESTNUT-BREASTED CUCKOO SOUTHERN CASSOWARY STRIATED HERON LEWIN’S RAIL CASPIAN TERN FAN-TAILED CUCKOO EMU NANKEEN NIGHT HERON PALE-VENTED BUSH-HEN WHITE-WINGED BLACK TERN BLACK-EARED CUCKOO AUSTRALASIAN GREBE GLOSSY IBIS BAILLON’S CRAKE ROCK DOVE HORSFIELD’S BRONZE- GREAT CRESTED GREBE AUSTRALIAN WHITE IBIS AUSTRALIAN SPOTTED CRAKE WHITE-HEADED PIGEON CUCKOO SHINING BRONZE-CUCKOO ORANGE-FOOTED STRAW-NECKED IBIS SPOTLESS CRAKE SPOTTED TURTLE-DOVE SCRUBFOWL LITTLE BRONZE-CUCKOO ROYAL SPOONBILL WHITE-BROWED CRAKE BROWN CUCKOO-DOVE AUSTRALIAN BRUSH-TURKEY EASTERN KOEL BLACK-NECKED STORK PURPLE SWAMPHEN EMERALD DOVE MAGPIE GOOSE CHANNEL-BILLED CUCKOO EASTERN OSPREY DUSKY MOORHEN COMMON BRONZEWING PLUMED WHISTLING-DUCK PHEASANT COUCAL PACIFIC BAZA EURASIAN COOT CRESTED PIGEON WANDERING WHISTLING- RUFOUS OWL DUCK BLACK-SHOULDERED KITE AUSTRALIAN BUSTARD SQUATTER PIGEON BLACK SWAN BARKING OWL SQUARE-TAILED KITE RED-BACKED BUTTON-QUAIL PEACEFUL DOVE RADJAH SHELDUCK * SOUTHERN BOOBOOK BLACK KITE LITTLE BUTTON-QUAIL BAR-SHOULDERED DOVE AUSTRALIAN WOOD DUCK * LESSER SOOTY OWL WHISTLING KITE RED-CHESTED BUTTON-QUAIL WOMPOO FRUIT-DOVE COTTON PYGMY-GOOSE * BRAHMINY KITE PAINTED BUTTON-QUAIL SUPERB FRUIT-DOVE * MASKED OWL GREEN PYGMY-GOOSE WHITE-BELLIED SEA-EAGLE LATHAM’S SNIPE ROSE-CROWNED FRUIT-DOVE EASTERN BARN OWL PACIFIC BLACK -

Of the Moreton Bay Region 25 Available (Some Apps Include Bird Calls And/Or Photographs)
Bellthorpe Birdwatching in Moreton Bay 1 South-East Queensland is one of Australia’s biodiversity hotspots. At the time of printing, 363 of Australia’s bird species (about 42%) 2 have been recorded in the Moreton Bay Region. This diversity is due to the subtropical location and the wide variety of habitats, including Woodford beaches, wetlands, grasslands, woodland, heathland, rainforest and large freshwater lakes. M1 20 General Birdwatching Tips 21 Go birdwatching about an hour after sunrise when most birds Toorbul Bribie are active. 24 Island Mount Mee Caboolture It is useful to get to know the calls of birds, especially the 3 23 22 common species. Please upload your sighting lists to an online database 4 M1 (birdata.birdlife.org or www.ebird.org) to help with MORETON BAY REGION conservation and management of Australia’s native birds. Deception Bay Resources: Use binoculars and have a field guide or phone app of the Moreton Bay Region 25 available (some apps include bird calls and/or photographs). Dayboro Narangba 26 9 27 A telescope is useful for shorebirds and at large water bodies. North Redcliffe 10 Lakes The Moreton Bay Region experiences a subtropical climate. It is important ’ 11 28 Petrie 33 M1 to wear sunscreen, protective clothing, a hat and carry insect repellent and Samsonvale 29 13 343231 30 water all year round. Closed shoes are essential at all birdwatching sites. 12 Strathpine 5 Common Moreton Bay Region Birds: These common birds are often Mount observed (in suitable habitats) throughout the Moreton Bay Region and Glorious 14 15 Albany Creek are not included in the individual site descriptions.