Decorative Magnolia Plants: a Comparison of the Content of Their Biologically Active Components Showing Antimicrobial Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
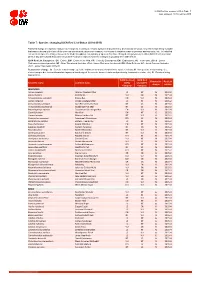
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Highly Water-Soluble Solid Dispersions of Honokiol: Preparation, Solubility, and Bioavailability Studies and Anti-Tumor Activity Evaluation
pharmaceutics Article Highly Water-Soluble Solid Dispersions of Honokiol: Preparation, Solubility, and Bioavailability Studies and Anti-Tumor Activity Evaluation Li Wang 1,2, Weiwei Wu 1,2, Lingling Wang 1,2, Lu Wang 1,2 and Xiuhua Zhao 1,2,* 1 College of Chemistry, Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin 150040, China; [email protected] (L.W.); [email protected] (W.W.); [email protected] (L.W.); [email protected] (L.W.) 2 Key Laboratory of Forest Plant Ecology, Northeast Forestry University, Ministry of Education, Harbin 150040, China * Correspondence: [email protected]; Tel.: +86-451-82191517; Fax: +86-451-82102082 Received: 18 September 2019; Accepted: 24 October 2019; Published: 1 November 2019 Abstract: Honokiol (HK), a well-tolerated natural product, has many multiple pharmacological activities. However, its poor water solubility and low bioavailability limit its clinical application and development. The aim of this research was to prepare the solid dispersion (SD) formulation of honokiol (HK) with poloxamer-188 (PLX) as the carrier, thereby improving its solubility and oral bioavailability. Firstly, by investigating the relationship between the addition amount of the PLX and the solubility of HK, and the effects of solid dispersions with different ratios of HK–PLX on the solubility of HK, we determined that the optimum ratio of PLX to HK was (1:4). Then, the HK–PLX (1:4) SD of HK was prepared using the solvent evaporation method. The morphology of the obtained HK–PLX (1:4) SD was different from that of free HK. The HK in the HK–PLX (1:4) SD existed in amorphous form and formed intermolecular hydrogen bonds with PLX. -

A Abdominal Aortic Aneurysm, 1405–1419 ABR. See Auditory
Index A Acute chest syndrome, 2501 Abdominal aortic aneurysm, 1405–1419 Acute cold stress, 378 ABR. See Auditory brainstem response (ABR) Acute cytokines, 3931 Abyssinones, 4064, 4073 Acute estrogen deprivation, 1336 Accelerated atherosclerosis, 3473, 3474, Acute ischemic stroke (AIS), 2195, 2198, 3476, 3478 2209, 2246–2252, 2265 Accidental hypothermia, 376 Acute kidney injury (AKI), 2582–2584, ACEIs. See Angiotensin-converting enzyme 2587–2591 inhibitors (ACEIs) Acute respiratory distress syndrome, 635 A549 cells, 177, 178 Acyl-CoA dehydrogenase (Acyl-CoADH), 307 Acetaldehyde, 651, 653, 1814 AD. See Alzheimer’s disease (AD) Acetaminophen, 630–631, 1825 Adaptation to intermittent hypoxia (AIH), N-acetyl-p-benzoquinone imine (NAPQI), 2213–2214 1771, 1773–1775 Adaptive immunity in interstitial lung N-Acetylaspartate, 2436 disease, 1616 Acetylcholine (ACh), 2277, 2278, 2280, 2281, Adaptive response, 1796–1798 2286, 2910, 2915 Adaptor protein SchA, 915 Acetylcholine receptor (ACh-R), 2910 AD assessment scale, cognitive scale Acetylcholinesterase (AChE) inhibitors, 2356, (ADAScog), 2362 2359, 3684 Ade´liepenguins (Pygoscelis adeliae),53 N-Acetyl-L-cysteine, 3588–3590, 3592 Adenine, 900, 902, 932 Acetyl radical, 2761 Adenine nucleotide translocase (ANT), 900, Acetylsalicylic acid (ASA), 2382–2383 902–905, 917, 918, 921, 922, 928 ACh. See Acetylcholine (ACh) Adenosine, 382, 387, 1951, 1953–1954, 2915 Acid b-oxidation, 798 Adenosine deaminase, 2436, 2440 Acidosis, 380–381, 3950, 3951, 3954–3956 Adenosine diphosphate (ADP), 3383–3386 Aconitase, -

(12) Patent Application Publication (10) Pub. No.: US 2006/014 1075A1 Talbott (43) Pub
US 2006O141075A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/014 1075A1 Talbott (43) Pub. Date: Jun. 29, 2006 (54) METHODS AND COMPOSITIONS FOR Publication Classification WEIGHT MANAGEMENT AND MOOD ENHANCEMENT (51) Int. Cl. A6IR 36/82 (2006.01) A6IR 36/752 (2006.01) (76) Inventor: Shawn M. Talbott, Draper, UT (US) A6II 3/522 (2006.01) A6II 3/56 (2006.01) Correspondence Address: A6IR 33/26 (2006.01) MACPHERSON KWOK CHEN & HEID LLP A 6LX 36/575 (2006.01) 1762 TECHNOLOGY DRIVE, SUITE 226 (52) U.S. Cl. ......................... 424/729; 424/736: 424/774; SAN JOSE, CA 95110 (US) 424/769; 514/263.31; 514/171; 424/646 (21) Appl. No.: 11/360,312 (57) ABSTRACT The present invention provides nutritional Supplement com (22) Filed: Feb. 23, 2006 positions and processes for treatment using nutritional Supplements that assist in weight management. One aspect Related U.S. Application Data of the invention comprises the inclusion in a single Supple ment constituent(s) that assist in reduction of a subjects (62) Division of application No. 10/895.253, filed on Jul. cortisol level, thermogenic constituent(s), and constituent(s) 20, 2004. that assist in stabilizing blood Sugar levels. Patent Application Publication Jun. 29, 2006 Sheet 1 of 4 US 2006/01.41075A1 Global Mood State (Profile of Mood States) 160 -8.6% 150 140 130 120 o Mood, Pre 110 Effect of a stress/cortisol-controlling dietary supplement on Global Mood States following 12 weeks of supplementation. P = placebo. S = Supplement. * = P <0.05 compared to Pre. -

Officinalis Var. Biloba and Eucommia Ulmoides in Traditional Chinese Medicine
Two Thousand Years of Eating Bark: Magnolia officinalis var. biloba and Eucommia ulmoides in Traditional Chinese Medicine Todd Forrest With a sense of urgency inspired by the rapid disappearance of plant habitats, most researchers are focusing on tropical flora as the source of plant-based medicines. However, new medicines may also be developed from plants of the world’s temperate regions. While working in his garden in the spring of English yew (Taxus baccata), a species common 1763, English clergyman Edward Stone was in cultivation. Foxglove (Digitalis purpurea), positive he had found a cure for malaria. Tasting the source of digitoxin, had a long history as the bark of a willow (Salix alba), Stone noticed a folk medicine in England before 1775, when a bitter flavor similar to that of fever tree (Cin- William Withering found it to be an effective chona spp.), the Peruvian plant used to make cure for dropsy. Doctors now prescribe digitoxin quinine. He reported his discovery to the Royal as a treatment for congestive heart failure. Society in London, recommending that willow EGb 761, a compound extracted from the be tested as an inexpensive alternative to fever maidenhair tree (Gingko biloba), is another ex- tree. Although experiments revealed that wil- ample of a drug developed from a plant native to low bark could not cure malaria, it did reduce the North Temperate Zone. Used as an herbal some of the feverish symptoms of the disease. remedy in China for centuries, ginkgo extract is Based on these findings, Stone’s simple taste now packaged and marketed in the West as a test led to the development of a drug used every treatment for ailments ranging from short-term day around the world: willow bark was the first memory loss to impotence. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

THE Magnoliaceae Liriodendron L. Magnolia L
THE Magnoliaceae Liriodendron L. Magnolia L. VEGETATIVE KEY TO SPECIES IN CULTIVATION Jan De Langhe (1 October 2014 - 28 May 2015) Vegetative identification key. Introduction: This key is based on vegetative characteristics, and therefore also of use when flowers and fruits are absent. - Use a 10× hand lens to evaluate stipular scars, buds and pubescence in general. - Look at the entire plant. Young specimens, shade, and strong shoots give an atypical view. - Beware of hybridisation, especially with plants raised from seed other than wild origin. Taxa treated in this key: see page 10. Questionable/frequently misapplied names: see page 10. Names referred to synonymy: see page 11. References: - JDL herbarium - living specimens, in various arboreta, botanic gardens and collections - literature: De Meyere, D. - (2001) - Enkele notities omtrent Liriodendron tulipifera, L. chinense en hun hybriden in BDB, p.23-40. Hunt, D. - (1998) - Magnolias and their allies, 304p. Bean, W.J. - (1981) - Magnolia in Trees and Shrubs hardy in the British Isles VOL.2, p.641-675. - or online edition Clarke, D.L. - (1988) - Magnolia in Trees and Shrubs hardy in the British Isles supplement, p.318-332. Grimshaw, J. & Bayton, R. - (2009) - Magnolia in New Trees, p.473-506. RHS - (2014) - Magnolia in The Hillier Manual of Trees & Shrubs, p.206-215. Liu, Y.-H., Zeng, Q.-W., Zhou, R.-Z. & Xing, F.-W. - (2004) - Magnolias of China, 391p. Krüssmann, G. - (1977) - Magnolia in Handbuch der Laubgehölze, VOL.3, p.275-288. Meyer, F.G. - (1977) - Magnoliaceae in Flora of North America, VOL.3: online edition Rehder, A. - (1940) - Magnoliaceae in Manual of cultivated trees and shrubs hardy in North America, p.246-253. -

Biologically Active Substances from Unused Plant Materials
Biologically active substances from unused plant materials Petra Lovecká1, Anna Macůrková1, Kateřina Demnerová1, Zdeněk Wimmer2 1Department of Biochemistry and Microbiology, 2Departement of Chemistry of Natural Compounds, University of Chemistry and Technology Prague Technická 3, 166 28 Prague 6, Czech Republic Keywords: Magnolia, honokiol, obovatol, biological activities. Presenting author email: [email protected] Natural products, such as plants extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug discoveries because of the unmatched availability of chemical diversity. Probably based on historical background we utilize only certain plant parts. In many cases there is not enough of required material or collection damages plant itself. The possible solution we could find in utilization of unused or waste plant parts. This work is focused on analysis of bioactive compounds content in different parts of medicinal plants such as genus Magnolia. Stem bark of these trees are part of Chinese traditional medicine. The collection of stem bark is devastating for the tree. Flowers and leaves from Magnolia tripetala, Magnolia obovata and their hybrids were separately extracted with 80% methanol. Resulting methanolic extract were subsequently fractionated with chloroform under acidic conditions and mixture of chloroform and methanol under basic conditions in term of increasing polarity (Harborne, 1998) into four fractions, neutral, moderately polar, basic and polar extract. Each extract was screened -

Review Article Small Molecules from Nature Targeting G-Protein Coupled Cannabinoid Receptors: Potential Leads for Drug Discovery and Development
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2015, Article ID 238482, 26 pages http://dx.doi.org/10.1155/2015/238482 Review Article Small Molecules from Nature Targeting G-Protein Coupled Cannabinoid Receptors: Potential Leads for Drug Discovery and Development Charu Sharma,1 Bassem Sadek,2 Sameer N. Goyal,3 Satyesh Sinha,4 Mohammad Amjad Kamal,5,6 and Shreesh Ojha2 1 Department of Internal Medicine, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, Abu Dhabi, UAE 2Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, Abu Dhabi, UAE 3DepartmentofPharmacology,R.C.PatelInstituteofPharmaceuticalEducation&Research,Shirpur,Mahrastra425405,India 4Department of Internal Medicine, College of Medicine, Charles R. Drew University of Medicine and Science, Los Angeles, CA 90059, USA 5King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia 6Enzymoics, 7 Peterlee Place, Hebersham, NSW 2770, Australia Correspondence should be addressed to Shreesh Ojha; [email protected] Received 24 April 2015; Accepted 24 August 2015 Academic Editor: Ki-Wan Oh Copyright © 2015 Charu Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The cannabinoid molecules are derived from Cannabis sativa plant which acts on the cannabinoid receptors types 1 and 2 (CB1 and CB2) which have been explored as potential therapeutic targets for drug discovery and development. Currently, there are 9 numerous cannabinoid based synthetic drugs used in clinical practice like the popular ones such as nabilone, dronabinol, and Δ - tetrahydrocannabinol mediates its action through CB1/CB2 receptors. -

NRDC: Generally Recognized As Secret
NRDC Report April 2014 Generally Recognized as Secret: Chemicals Added to Food in the United States Tom Neltner, J.D., Maricel Maffini, Ph.D. Natural Resources Defense Council In April 2014, the Natural Resources Defense Council (NRDC) released a report raising concerns about a loophole in the Food Additives Amendment of 1958 for substances designated by food manufacturers as “generally recognized as safe” (GRAS). The report identified 56 companies that appeared to market 275 chemicals for use in food based on undisclosed GRAS safety determinations. For each chemical we identified in this study, we did not find evidence that FDA had cleared them for use in food. The 1958 law exempted from the formal, extended FDA approval process common food ingredients like vinegar and vegetable oil whose use qualifies as GRAS. It may have appeared reasonable at the time, but that exemption has been stretched into a loophole that has swallowed the law. The exemption allows manufacturers to make safety determinations that the uses of their newest chemicals in food are safe without notifying the FDA. The agency’s attempts to limit these undisclosed GRAS determinations by asking industry to voluntarily inform the FDA about their chemicals are insufficient to ensure the safety of our food in today’s global marketplace with a complex food supply. Furthermore, no other developed country in the world has a system like GRAS to provide oversight of food ingredients. In Table 1 and 2 of the report, NRDC identified the 56 companies and the number of chemicals that each company appeared to market as GRAS without FDA clearance. -

Herbal Remedies and Their Possible Effect on the Gabaergic System and Sleep
nutrients Review Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep Oliviero Bruni 1,* , Luigi Ferini-Strambi 2,3, Elena Giacomoni 4 and Paolo Pellegrino 4 1 Department of Developmental and Social Psychology, Sapienza University, 00185 Rome, Italy 2 Department of Neurology, Ospedale San Raffaele Turro, 20127 Milan, Italy; [email protected] 3 Sleep Disorders Center, Vita-Salute San Raffaele University, 20132 Milan, Italy 4 Department of Medical Affairs, Sanofi Consumer HealthCare, 20158 Milan, Italy; Elena.Giacomoni@sanofi.com (E.G.); Paolo.Pellegrino@sanofi.com (P.P.) * Correspondence: [email protected]; Tel.: +39-33-5607-8964; Fax: +39-06-3377-5941 Abstract: Sleep is an essential component of physical and emotional well-being, and lack, or dis- ruption, of sleep due to insomnia is a highly prevalent problem. The interest in complementary and alternative medicines for treating or preventing insomnia has increased recently. Centuries-old herbal treatments, popular for their safety and effectiveness, include valerian, passionflower, lemon balm, lavender, and Californian poppy. These herbal medicines have been shown to reduce sleep latency and increase subjective and objective measures of sleep quality. Research into their molecular components revealed that their sedative and sleep-promoting properties rely on interactions with various neurotransmitter systems in the brain. Gamma-aminobutyric acid (GABA) is an inhibitory neurotransmitter that plays a major role in controlling different vigilance states. GABA receptors are the targets of many pharmacological treatments for insomnia, such as benzodiazepines. Here, we perform a systematic analysis of studies assessing the mechanisms of action of various herbal medicines on different subtypes of GABA receptors in the context of sleep control. -
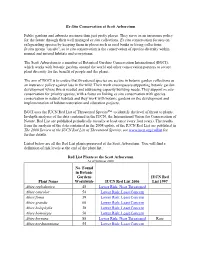
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details.