Federal Register/Vol. 85, No. 211/Friday, October 30, 2020/Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
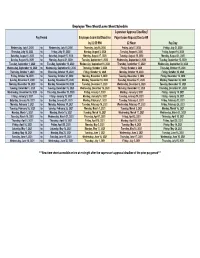
Copy of 2020-2021 Semi-Monthly Calendar.Xlsx
Employee Time Sheet/Leave Sheet Schedule Supervisor Approval Deadline/ Pay Period Employee Submittal Deadline Paper Leave Request Due to HR by 11:59 PM 12 Noon Pay Day Wednesday, July 1, 2020 to Wednesday, July 15, 2020 Thursday, July 16, 2020 Friday, July 17, 2020 Friday, July 31, 2020 Thursday, July 16, 2020 to Friday, July 31, 2020 Monday, August 3, 2020 Tuesday, August 4, 2020 Friday, August 14, 2020 Saturday, August 1, 2020 to Saturday, August 15, 2020 Monday, August 17, 2020 Tuesday, August 18, 2020 Monday, August 31, 2020 Sunday, August 16, 2020 to Monday, August 31, 2020 Tuesday, September 1, 2020 Wednesday, September 2, 2020 Tuesday, September 15, 2020 Tuesday, September 1, 2020 to Tuesday, September 15, 2020 Wednesday, September 16, 2020 Thursday, September 17, 2020 Wednesday, September 30, 2020 Wednesday, September 16, 2020 to Wednesday, September 30, 2020 Thursday, October 1, 2020 Friday, October 2, 2020 Thursday, October 15, 2020 Thursday, October 1, 2020 to Thursday, October 15, 2020 Friday, October 16, 2020 Monday, October 19, 2020 Friday, October 30, 2020 Friday, October 16, 2020 to Saturday, October 31, 2020 Monday, November 2, 2020 Tuesday, November 3, 2020 Friday, November 13, 2020 Sunday, November 1, 2020 to Sunday, November 15, 2020 Monday, November 16, 2020 Tuesday, November 17, 2020 Monday, November 30, 2020 Monday, November 16, 2020 to Monday, November 30, 2020 Tuesday, December 1, 2020 Wednesday, December 2, 2020 Tuesday, December 15, 2020 Tuesday, December 1, 2020 to Tuesday, December 15, 2020 Wednesday, December -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
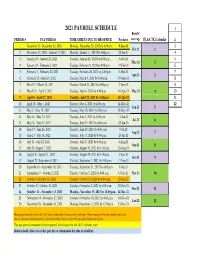
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

2018 - 2019 Days of Rotation Calendar
2018 - 2019 DAYS OF ROTATION CALENDAR Day # Date Rotation Day Type Notes Day # Date Rotation Day Type Notes Saturday, October 13, 2018 Sunday, October 14, 2018 Monday, September 3, 2018 Holiday/Vaca Labor Day 27 Monday, October 15, 2018 Day 3 In Session 1 Tuesday, September 4, 2018 Day 1 In Session 28 Tuesday, October 16, 2018 Day 4 In Session 2 Wednesday, September 5, 2018 Day 2 In Session 29 Wednesday, October 17, 2018 Day 5 In Session 3 Thursday, September 6, 2018 Day 3 In Session 30 Thursday, October 18, 2018 Day 6 In Session 4 Friday, September 7, 2018 Day 4 In Session 31 Friday, October 19, 2018 Day 1 In Session Saturday, September 8, 2018 Saturday, October 20, 2018 Sunday, September 9, 2018 Sunday, October 21, 2018 Monday, September 10, 2018 Day Holiday/Vaca Rosh Hashanah 32 Monday, October 22, 2018 Day 2 In Session 5 Tuesday, September 11, 2018 Day 5 In Session 33 Tuesday, October 23, 2018 Day 3 In Session 6 Wednesday, September 12, 2018 Day 6 In Session 34 Wednesday, October 24, 2018 Day 4 In Session 7 Thursday, September 13, 2018 Day 1 In Session 35 Thursday, October 25, 2018 Day 5 In Session 8 Friday, September 14, 2018 Day 2 In Session 36 Friday, October 26, 2018 Day 6 In Session Saturday, September 15, 2018 Saturday, October 27, 2018 Sunday, September 16, 2018 Sunday, October 28, 2018 9 Monday, September 17, 2018 Day 3 In Session 37 Monday, October 29, 2018 Day 1 In Session 10 Tuesday, September 18, 2018 Day 4 In Session 38 Tuesday, October 30, 2018 Day 2 In Session Wednesday, September 19, 2018 Day Holiday/Vaca Yom Kippur 39 Wednesday, October 31, 2018 Day 3 In Session 11 Thursday, September 20, 2018 Day 5 In Session 40 Thursday, November 1, 2018 Day 4 In Session 12 Friday, September 21, 2018 Day 6 In Session 41 Friday, November 2, 2018 Day 5 In Session Saturday, September 22, 2018 Saturday, November 3, 2018 Sunday, September 23, 2018 Sunday, November 4, 2018 13 Monday, September 24, 2018 Day 1 In Session 42 Monday, November 5, 2018 Day 6 In Session 14 Tuesday, September 25, 2018 Day 2 In Session Tuesday, November 6, 2018 Prof Dev. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

October 30, 1956 Working Notes from the Session of the CPSU CC Presidium on 30 October 1956
Digital Archive digitalarchive.wilsoncenter.org International History Declassified October 30, 1956 Working Notes from the Session of the CPSU CC Presidium on 30 October 1956 Citation: “Working Notes from the Session of the CPSU CC Presidium on 30 October 1956,” October 30, 1956, History and Public Policy Program Digital Archive, TsKhSD, F. 3, Op. 12, D. 1006, Ll. 6-14, compiled by V. N. Malin. Published in CWIHP Bulletin 8-9, pp. 392-393. http://digitalarchive.wilsoncenter.org/document/113647 Summary: The Presidium decides to promulgate a declaration on Hungary in which Soviet withdrawal and relations with the new government will be addressed. Members discuss the language of the new declaration and the advice of the CPC CC regarding the status of Soviet troops. The declaration is also intended to address the broader crisis in Soviet relations with people’s democracies. Original Language: Russian Contents: English Translation Working Notes from the Session of the CPSU CC Presidium on 30 October 1956(1) (Re: Point 1 of Protocol No. 49)(2) Those Taking Part: Bulganin, Voroshilov, Molotov, Kaganovich, Saburov, Brezhnev, Zhukov, Shepilov, Shvernik, Furtseva, Pospelov On the Situation in Hungary Information from Cdes. Mikoyan and Serov is read aloud.(3) Cde. Zhukov provides information about the concentration of mil.-transport aircraft in the Vienna region.(4) Nagy is playing a double game (in Malinin's opinion). Cde. Konev is to be sent to Budapest.(5) On Discussions with the Chinese comrades. (6) (Khrushchev) We should adopt a declaration today on the withdrawal of troops from the countries of people's democracy (and consider these matters at a session of the Warsaw Pact), taking account of the views of the countries in which our troops are based. -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

COVID-19 Update October 30, 2020
COVID-19 Update October 30, 2020 As of October 29, 2020, at 8:30 PM, the total of laboratory-confirmed and probable COVID-19 cases reported among Connecticut residents is 71207, including 67519 laboratory-confirmed and 3688 probable cases. Three hundred twenty-nine patients are currently hospitalized with laboratory- confirmed COVID-19. There have been 4616 COVID-19-associated deaths. In Connecticut during the early months of this pandemic, it became increasingly clear that it would be necessary to track probable COVID-19 cases and deaths, in addition to laboratory-confirmed (RT-PCR) cases and deaths. This was needed to better measure the burden and impact of this disease in our communities and is now part of the national surveillance case definition for COVID-19. Prior to June 1, probable and confirmed cases were reported together. Overall Summary Total** Change Since Yesterday COVID-19 Cases 71207 +761 COVID-19-Associated Deaths 4616 +7 Patients Currently Hospitalized with COVID-19 329 +8 COVID-19 PCR Tests Reported 2284896 +30554 **Includes confirmed plus probable cases COVID-19 Cases and Associated Deaths by County of Residence As of 10/29/20 8:30pm. COVID-19 Cases COVID-19-Associated Deaths County Confirmed Probable Confirmed Probable Fairfield County 22737 1418 1120 316 Hartford County 17297 887 1158 323 Litchfield County 2158 147 123 21 Middlesex County 1922 96 158 39 New Haven County 16489 863 970 158 New London County 3695 108 108 35 Tolland County 1648 132 53 15 Windham County 1449 20 18 1 Pending address validation 124 17 0 0 Total 67519 3688 3708 908 National COVID-19 statistics and information about preventing spread of COVID-19 are available from the Centers for Disease Control and Prevention. -

2016 7 Day Working Days Calendar
2016 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2016 306 Saturday, January 2, 2016 307 Sunday, January 3, 2016 308 Monday, January 4, 2016 309 Tuesday, January 5, 2016 310 Wednesday, January 6, 2016 311 Thursday, January 7, 2016 312 Friday, January 8, 2016 313 Saturday, January 9, 2016 314 Sunday, January 10, 2016 315 Monday, January 11, 2016 316 Tuesday, January 12, 2016 317 Wednesday, January 13, 2016 318 Thursday, January 14, 2016 319 Friday, January 15, 2016 320 Saturday, January 16, 2016 321 Sunday, January 17, 2016 322 Monday, January 18, 2016 323 Tuesday, January 19, 2016 324 Wednesday, January 20, 2016 325 Thursday, January 21, 2016 326 Friday, January 22, 2016 327 Saturday, January 23, 2016 328 Sunday, January 24, 2016 329 Monday, January 25, 2016 330 Tuesday, January 26, 2016 331 Wednesday, January 27, 2016 332 Thursday, January 28, 2016 333 Friday, January 29, 2016 334 Saturday, January 30, 2016 335 Sunday, January 31, 2016 336 Monday, February 1, 2016 337 Tuesday, February 2, 2016 338 Wednesday, February 3, 2016 339 Thursday, February 4, 2016 340 Date Number of the Calendar Date Friday, February 5, 2016 341 Saturday, February 6, 2016 342 Sunday, February -

OCTOBER 30 OCTOBER 31 NOVEMBER 1 NOVEMBER 2 10 A.M
MONDAY WEDNESDAY THURSDAY FRIDAY SATURDAY OCTOBER 28 OCTOBER 30 OCTOBER 31 NOVEMBER 1 NOVEMBER 2 10 a.m. - 9 p.m. 10 a.m. - 9 p.m. 10 a.m. - 9 p.m. 10 a.m. - 9 p.m. 10 a.m. - 9 p.m. SOUTHERN GOSPEL JUBILEE PERFORMANCES DP’s Celebrity Theater 45 Gold City DP’s Celebrity Theater is closed in preparation for Dollywood’s min (Map #1) PASS 1:00, 3:00, 5:00 Christmas in the Smokies! The Kingdom Heirs The Kingdom Heirs The Kingdom Heirs The Kingdom Heirs 10:15, 12:00 10:15, 12:00 10:15, 12:00 10:15, 12:00 The Pines Theater 40 Jeff & Sheri Easter min (Map #129) PASS The Freemans The Ball Brothers The Ball Brothers Jeff & Sheri Easter 12:30, 2:30, 4:30 3:30, 5:30, 7:30 3:30, 5:30, 7:30 3:30, 5:30, 7:30 3:30, 5:30, 7:30 The Porter Family JP Miller Isbell Family The Roarks PromisedLand 40 Quartet min The Back Porch Theater 10:45, 12:00, 1:15 10:45, 12:00, 1:15 10:45, 12:00, 1:15 10:45, 12:00, 1:15 10:45, 12:00, 1:15 (Map #111) 40 Heartline Isbell Family Battle Cry Troy Burns Family Michael Wayne min Smith 3:45, 5:00, 6:15 3:45, 5:00, 6:15 3:45, 5:00, 6:15 3:45, 5:00, 6:15 3:45, 5:00, 6:15 King James Boys Gloryway Chronicle Answered Prayer 11:00, 12:15, 1:30 11:00, 12:15, 1:30 11:00, 12:15, 1:30 11:00, 12:15, 1:30 The Valley Theater 40 Cami Shrock min (Map #53) 11:30, 1:00, 2:30 Gloryway The Hinson Family Answered Prayer Chronicle 4:00, 5:15, 7:00 4:00, 5:15, 7:00 4:00, 5:15, 7:00 4:00, 5:15, 7:00 WILDWOOD GROVE FEATURES Wild Roots Band 20 11:15, 12:15, 11:15, 12:15, 11:15, 12:15, 11:15, 12:15, 11:15, 12:15, Till & Harvest Patio (Map #151) min 2:00, -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3,