Jumping Behaviour in a Gondwanan Relict Insect (Hemiptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemiptera: first Record for an Australian Lophopid (Hemiptera, Lophopidae)
Australian Journal of Entomology (2007) 46, 129–132 Historical use of substrate-borne acoustic production within the Hemiptera: first record for an Australian Lophopid (Hemiptera, Lophopidae) Adeline Soulier-Perkins,1* Jérôme Sueur2 and Hannelore Hoch3 1Muséum National d’Histoire Naturelle, Département Systématique et Évolution, USM 601 MNHN & UMR 5202 CNRS, Case Postale 50, 45, Rue Buffon, F-75005 Paris, France. 2NAMC-CNRS UMR 8620, Bât. 446, Université Paris XI, F-91405 Orsay Cedex, France. 3Museum für Naturkunde, Institut für Systematische Zoologie, Humboldt-Universität zu Berlin Invalidenstr. 43, D- 10115 Berlin, Germany. Abstract Here the first record of communication through substrate-borne vibrations for the Lophopidae family is reported. The signals from Magia subocellata that the authors recorded were short calls with a decreasing frequency modulation. Acoustic vibrations have been observed for other families within the Hemiptera and a scenario concerning the historical use of vibrational communication within the Hemiptera is tested using a phylogenetic inference. The most parsimonious hypothesis suggests that substrate-borne communication is ancestral for the hemipteran order and highlights the groups for which future acoustic research should be undertaken. Key words Cicadomorpha, Coleorrhyncha, evolutionary scenario, Heteroptera, Sternorrhyncha, substrate vibration. INTRODUCTION Lophopidae migrating into America via the Bering land bridge. Some other ancestors of the extant groups moved onto Many animals have been recently recognised for their ability newly emerging land in the Pacific, expanding their distribu- to communicate through substrate-borne vibrations (Hill tion as far east as the Samoan Islands, and as far south as 2001). While elephants produce vibrations transmitted by the Australia (Soulier-Perkins 2000). -

Order 4 Hemiptera Derivation: (Gk. Hemi=Half; Petron=A Wing
Order 4 Hemiptera Derivation: (Gk. Hemi=half; petron=a wing) Common names: (Bugs, aphids, hoppers, etc.) Metamorphosis: Incomplete (Paurometabolous) - Distribution: Worldwide Number of families: 134 Bugs, which comprise about 8 % of all insect species, are the largest and most successful of the Exopterygote orders. They range from minute, wingless, scale insects, hardly resembling insects at all, to giant water bugs with raptorial front legs capable of catching fish and frogs. Almost every type of terrestrial and freshwater habitat has a particular and characteristic bug fauna, and ocean striders of genus Halobates (Gerridae) can found on the sea, hundreds of miles from the land. In the past, the order was split into two large suborders, the Heteroptera (the true land and water bugs) and the Homoptera (the aphids, scale insect, hoppers, plant lice, mealy bugs, and related species), on the base of wing characteristics. Two pairs of wings are usually present and in heteropterans, the basal part of the front wing is toughened, leaving a membranous region on the tip. In homopterans, the front wings and hind wings may be memberanous, or the front wings may be entirely toughened. Modern classification recognize four distinct suborders: the Coleorrhyncha (beetle bugs, a single family of bugs found in the southern hemisphere); Heteroptera (73 families of true bugs); Auchenorrhyncha (31 families of the plantoppers, leafhoppers, treehoppers, froghoppers, lantern bugs and cicadas); Sternorrhyncha (29 families of whiteflies, aphids, conifer woolly aphids, scale insects, mealy bugs, phylloxerans, jumping plant lice). All Auchenorrhyncha, Coleorrhyncha and Sternorrhyncha are herbivorous, feeding on the sap or cell contents of vascular plants. -

A New Family of Coreoidea from the Lower Cretaceous Lebanese Amber (Hemiptera: Pentatomomorpha)
P O L I S H JOUR NAL OF ENTOMOLO G Y POLSKIE PISMO ENTOMOL OGICZ N E VOL. 80: 627-644 Gdynia 31 December 2011 DOI: 10.2478/v10200-011-0049-5 A new family of Coreoidea from the Lower Cretaceous Lebanese Amber (Hemiptera: Pentatomomorpha) DANY AZAR1, ANDRÉ NEL2, MICHAEL S. ENGEL3, 4, ROMAIN GARROUSTE2, ARMAND MATOCQ2 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, PO box 26110217, Fanar – Matn, Lebanon, e-mail: [email protected]; 2CNRS UMR 7205, CP 50, Entomologie, Muséum National d'Histoire Naturelle, 45 rue Buffon, F-75005 Paris, France, e-mails: [email protected], [email protected], [email protected]; 3Division of Entomology (Paleoentomology), Natural History Museum, University of Kansas, Lawrence, KS 66049-2811, USA, e-mail: [email protected]; 4Department of Ecology & Evolutionary Biology, 1501 Crestline Drive – Suite 140, University of Kansas, Lawrence, KS 66049-2811, USA ABSTRACT. A new genus and species, Yuripopovina magnifica, belonging to a new coreoid family, Yuripopovinidae (Hemiptera: Pentatomomorpha), is described and illustrated from the Lower Cretaceous amber of Lebanon. The species represents the first definitive Mesozoic record for the Coreoidea. A cladistic analysis of Coreoidea, including the new family, is undertaken. KEY WORDS: Pentatomomorpha, Coreoidea, Yuripopovinidae, fam. n., gen. n., sp. n., Lebanon, phylogeny. INTRODUCTION The Pentatomomorpha with its 14 000 known living species (WEIRAUCH & SCHUH 2011) is the second largest of the seven heteropteran infraorders (SCHAEFER 1993, ŠTYS & KERZHNER 1975) (Enicocephalomorpha, Dipsocoromorpha, Gerromorpha, Nepomorpha, Leptodomorpha, Cimicomorpha, and Pentatomorpha). Most authors recognize five superfamilies within Pentatomomorpha, but there remains controversy regarding the 628 Polish Journal of Entomology 80 (4) composition of these superfamilies (SCHAEFER 1993, ŠTYS 1961). -

The Coleorrhyncha (Insecta: Hemiptera) of the European Jurassic, with a Description of a New Genus from the Toarcian of Luxembourg
Volumina Jurassica, 2011, iX: 3–20 The Coleorrhyncha (Insecta: Hemiptera) of the European Jurassic, with a description of a new genus from the Toarcian of Luxembourg Jacek SZWEDO1 Key words: Indutionomarus treveriorum gen. et sp. nov., Mesocimex anglicus (Yu. Popov, Dolling et Whalley) comb. nov., Toarcian Oceanic Anoxic Event, taxonomy, phylogeny, palaeoclimate, palaeoenvironment. Abstract. The fossil record of the Coleorrhyncha goes back to the Upper Permian. In recent faunas only members of the Peloridiidae are present, restricted in distribution to the Southern Hemisphere. These insects were more diversified in the past, and though their fossil re- cord in the Jurassic is restricted to the Northern Hemisphere, it comprises the families Progonocimicidae and Karabasiidae. The subfamily Progonocimicinae, present in the Jurassic strata of Europe and Asia is a declining lineage. The subfamily Cicadocorinae originated at the Triassic/Jurassic boundary and became dominant during Jurassic times. A review of Coleorrhyncha from European fossil sites is given, with taxonomic and phylogenetic problems highlighted. Their occurrence is linked to a very humid and warm climate, which is in agreement with independent data indicating greenhouse conditions in the atmospheric system and anoxia in the oceans at that time (Toarcian-Oceanic Anoxic Event – T-OAE) and coeval greenhouse climate on land. A new genus and species of the Progonocimicinae – Indutionomarus treveriorum gen. et sp. nov. is described, based on a specimen from the Lower Toarcian of Bascharage, Luxembourg, Western Europe. It is the first record of theColeorrhyncha from this locality. The morphological features of the new genus in respect to other Progonocimicidae, and its phylogenetic importance, are discussed. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

Hemiptera, Prosorrhyncha) with Special Reference to the Pregenital Abdominal Structure1
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Justification for the Aradimorpha as an infraorder of the suborder Heteroptera (Hemiptera, Prosorrhyncha) with Special Reference to the Pregenital Abdominal Structure1 M.H. SWEET Abstract: Aradomorpha SWEET 1996 is replaced with Aradimorpha because of homonymy with Arado- morpha CHAMPION 1899, a genus of Reduviidae. The Aradimorpha differ from the Pentatomomorpha s.s. and the Leptopodomorpha in having a plesiomorphic connexivum of dorsal epipleurites and ventral hy- popleurites rather than having the connexivum turned over so that the hypopleurites are dorsalized and the epipleurites folded into the abdomen. In most Aradimorpha, in both males and females, sterna 3 to 7 are free with intersegmental conjunctiva; terga 1-2 and 3 to 6 are united, but all epipleurites are free. In the Pentatomomorpha at least abdominal sterna 2 to 4 in females and sterna 2 to 5 in males are uni- ted or fused without conjunctiva. In some aradids the hypopleurites are united or fused with the sterna, but hypopleurite 2 is usually free. Sternum 2 is sometimes united to fused with sternum 1 and the meta- sternum. The abdominal spiracles in the Aradimorpha are ventral on the hypopleurites, although some- times very lateral in position on the hypopleurites, with the exception of the Chinamyersiini in which spiracles 4, 5 and 6 are dorsal on the epipleurites in Chinamyersia, and 5 and 6 dorsal in Gnostocoris, whi- le in the Tretocorini (Tretocoris and Kumaressa) spiracle 2 seems dorsal but is actually very lateral on the hypopleurite. In the Termitaphididae, epipleurites and hypopleurites are distinct, forming mobile lateral abdominal lobes. -
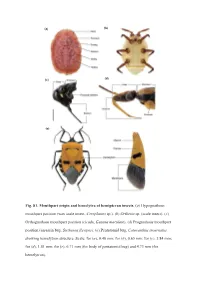
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

The Phylogeny of the Homoptera
PAP. & Prwe. ROY. Soc. TASMANIA. 1941 (35TH JU.I\;E j :i942.) 37 The Phylogeny of the Homoptera By ,T. W. EVANS (Read lOth ~ovember, HJ41) The question of the phylogeny of the Homoptera does not remain unsettled for want of discussion, as several authors have expressed most definite, though often contradictory, views on the subject. A few years ago a Bulletin entitled , The Phylogeny of the Hemiptera based on the Study of the Head Capsule' was published in the United States (Spooner, 1938). This work is no mere short paper, but a considerable contribution of over one hundred pages, containing close on four hundred figures. For this reason, quite apart from its obvious worth as a contribution to the comparative morphology of insects, it merits close attention. It consists of two parts; the first part deals with the Homoptera and the second with the Heteroptera, and at the end of each part the conclusions reached by the author with respect to phylogeny are expressed as trees. As far as the Heteroptera are concerned, no comment is offered, but the present paper has been written to dispute certain conclusions reached by Spooner regarding the inter-relationships of the Homoptera, and to present alternative proposals. In the phylogenetic tree for the Homoptera three main lines of descent are shown radiating from the Protohomoptel'ous stem. Two of these give rise to the Fulgoridae and Peloridiidae respectively and the third to the Cercopidae, all the several other groups of Homoptel'a, apart from those mentioned above, then being derived direct from the Cercopidae. -

Hemiptera, Coleorrhyncha, Peloridiidae)
An anomalous moss-bug from Southern Chile and notes on Pantinia darwini (Hemiptera, Coleorrhyncha, Peloridiidae) Autor(en): Burckhardt, Daniel / Cekalovic K., Tomas Objekttyp: Article Zeitschrift: Mitteilungen der Schweizerischen Entomologischen Gesellschaft = Bulletin de la Société Entomologique Suisse = Journal of the Swiss Entomological Society Band (Jahr): 75 (2002) Heft 1-2 PDF erstellt am: 25.09.2021 Persistenter Link: http://doi.org/10.5169/seals-402817 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte -

Yuri Popov — As We Remember Him
Russian Entomol. J. 26(3): 289–298 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2017 Personalia Yuri Popov — as we remember him Dr. Yuri Popov, born 5 March 1936, passed away 16 Recent fauna in his work on fossils, an effort that was November 2016. Upon graduation from the Entomology informed by his early and continuing interest in the study Department of Moscow State University, he joined the of the living Heteroptera. His published works will serve Arthropoda Lab of the Paleontological Institute, where as a lasting reminder of the energy he put into fieldwork he studied fossil and living true bugs and their kin and and the detailed study of the specimens derived from it. became a major expert in that area. He was a man of May he rest in peace. many talents and had lots of friends all over the world. The few flashbacks collected here are but a small tribute Robin Wootton to his memory. Although we have seldom met in the last five de- cades, I have always considered Yuri Alexandrovich to Randall T. Schuh be one of my greatest friends. I came to work at PIN in Yuri and I crossed paths two times. First, in my office 1963, on one of the first exchange fellowships between at the American Museum of Natural History, sometime the Soviet Academy of Sciences and the Royal Society. in the 1990s. We had a conversation about bugs and got The PIN staff were very welcoming, though Elena acquainted. When he left, I felt like I had a new friend. Ernestovna Bekker-Migdisova and Alexander Grig- The other time was at the second meeting of the Interna- orievich Sharov both tended to lecture me about the tional Heteropterists’ Society in St.