ECAT Science How the E-Cat Works
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Qu'est-Ce Que Le Temps ? TABLE DES MATIERES Qu'est-Ce Que Le Temps ?
Retrouvez cette oeuvre et beaucoup d'autres sur http://www.atramenta.net Qu'est-ce que le temps ? TABLE DES MATIERES Qu'est-ce que le temps ?.............................................................................1 Qu’est-ce que le temps ?......................................................................2 Che cos’è il tempo ? Quid est enim tempus ? ¿Qué es el tiempo ? Çfarë është koha ? What is time ? Was ist Zeit ? Wat is tijd ? Što je vrijeme ? Zaman Nedir ?Что такое время ? 什么是时间 ?.....................................................................2 Avant-propos.......................................................................................7 Urgences (quand le temps est compressé).........................................10 Développement durable.....................................................................26 Cause première… cause toujours !..........................................60 Introduction.......................................................................................94 Les âges de l’univers (et promenade dans les calendriers)................96 Les âges du Soleil............................................................................137 Les âges de la Terre.........................................................................144 Les âges de l’homme et de la femme..............................................154 Les âges des autres êtres vivants.....................................................175 Les âges des atomes et des particules..............................................182 -
Tests Find Rossi's E-Cat Has an Energy Density at Least 10 Times Higher Than Any Conventional Energy Source 23 May 2013, by Lisa Zyga
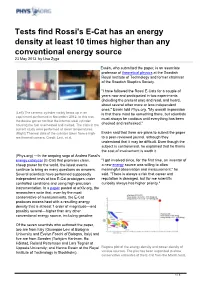
Tests find Rossi's E-Cat has an energy density at least 10 times higher than any conventional energy source 23 May 2013, by Lisa Zyga Essén, who submitted the paper, is an associate professor of theoretical physics at the Swedish Royal Institute of Technology and former chairman of the Swedish Skeptics Society. "I have followed the Rossi E-Cats for a couple of years now and participated in two experiments (including the present one) and read, and heard, about several other more or less independent ones," Essén told Phys.org. "My overall impression (Left) The ceramic cylinder visibly heats up in an is that there must be something there, but scientists experiment performed in November 2012. In this test, must always be cautious until everything has been the device got so hot that the internal steel cylinder housing the fuel overheated and melted. The trials in the checked and rechecked." current study were performed at lower temperatures. (Right) Thermal data of the cylinder taken from a high- Essén said that there are plans to submit the paper res thermal camera. Credit: Levi, et al. to a peer-reviewed journal, although they understand that it may be difficult. Even though the subject is controversial, he explained that he thinks the cost of involvement is worth it. (Phys.org) —In the ongoing saga of Andrea Rossi's energy catalyzer (E-Cat) that promises clean, "I got involved since, for the first time, an inventor of cheap power for the world, the latest events a new energy source was willing to allow continue to bring as many questions as answers. -

Cold Fusion Died 25 Years Ago, but the Research Lives on | November 7, 2016 Issue - Vol
11/7/2016 Cold fusion died 25 years ago, but the research lives on | November 7, 2016 Issue - Vol. 94 Issue 44 | Chemical & Engineering News 64.124.29.44 Volume 94 Issue 44 | pp. 34-39 Issue Date: November 7, 2016 COVER STORY Cold fusion died 25 years ago, but the research lives on Scientists continue to study unusual heat-generating effects, some hoping for vindication, others for and an eventual payday By Stephen K. Ritter Some 7,000 people attended a hastily organized cold fusion session at the ACS national meeting in Dallas in 1989, hopeful that word of the newly announced phenomenon was true. Credit: James Krieger/C&EN Howard J. Wilk is a long-term unemployed In brief synthetic organic chemist living in In 1989, the scientific world was turned upside down when two researchers announced they Philadelphia. Like many pharmaceutical had tamed the power of nuclear fusion in a researchers, he has suffered through the drug simple electrolysis cell. The excitement quickly died when the scientific community came to a industry’s R&D downsizing in recent years and consensus that the findings weren’t real—“cold fusion” became a synonym for junk science. In now is underemployed in a nonscience job. the quarter-century since, a surprising number of researchers continue to report unexplainable With extra time on his hands, Wilk has been excess heat effects in similar experiments, and several companies have announced plans to tracking the progress of a New Jersey-based commercialize technologies, hoping to company called Brilliant Light Power revolutionize the energy industry. -

COLD NUCLEAR FUSION from Pons & Fleischmann to Rossi's E-Cat
COLD NUCLEAR FUSION from Pons & Fleischmann to Rossi's E-Cat by Martin Bier Twenty-two years have passed since Pons and Fleischmann held their legendary press conference. Presumably, they had realized cold fusion. But it became a classic case of pride before the fall. A few months later, after the results appeared irreproducible, the American Physical Society and the authoritative journals declared it pseudoscience. Nevertheless, cold fusion never totally disappeared. Money has continued to be poured into it and researchers are still working on it. Recently, there has been commotion over an alleged "breakthrough" by Andrea Rossi with his E-Cat. But there are indications that Rossi's E-Cat is a sham. ! PONS EN FLEISCHMANN Martin Fleischmann (1927) was an accomplished British elektrochemist. He had been president of the International Society of Electrochemistry for two years. In 1986, he was allowed to join the Fellowship of the Royal Society. After 1983, he no longer had any teaching duties at the University of Southampton and started spending a lot of time doing research at the University of Utah. Stanley Pons (1943) was from Valdese, North Carolina. He interrupted his chemistry studies for eight years to help run the family business. But in 1975 he picked it up again and in 1978 he received his Ph.D. from the University of Southampton. In 1989, he was head of the chemistry department at the The front cover of Time on May 8, 1989.! University of Utah in Salt Like City. ! 1 The two scientists would have preferred to just publish their results in a scientific journal. -

Press Releases
HOW ROSSI COLD FUSION TESTS MISLED THE WORLD’S SCIENTISTS INADVERTENT MISWIRING OF LEADS IS THE CAUSE All around the world, thousands of people have been heralding the introduction of the Rossi E-CAT energy catalyzer device which claims to generate more energy output than is input due to low energy nuclear reaction. What makes this claim different is that at least fifteen scientists from around the world, including from NASA, have lent some support for it, after witnessing a demonstration or analyzing the results. They generally concluded that the output power was apparently much larger than the input, and only nuclear reactions could account for the difference. However, Dick Smith, patron of Australian Skeptics, says, “It would be great if it’s true, but it’s more likely just a misconnection of the power lead.” Smith was approached in December to invest $200,000 in this scheme to provide almost free energy and thus save the planet from climate change. He sent aerospace engineer Ian Bryce of Australian Skeptics to an investors’ meeting on the NSW North Coast on January 13 to investigate. Smith says, “If one of the wires in the three-core power lead was accidentally misconnected, the actual measurements of current witnessed by two Swedish scientists would not be the total power going into the reactor, and there would be an apparent power gain. One of the scientists who observed an earlier test has now agreed this could be so.” Bryce found that in all six published tests up to July, a misconnected earth lead could funnel in up to 3 kilowatts, thus bypassing the power meters used, and accounting for all the measured output power in the form of steam. -

130 Electrical Energy Innovations
130 Electrical Energy Innovations Gary Vesperman (Author) Advisor to Sky Train Corporation www.skytraincorp.com 588 Lake Huron Lane Boulder City, NV 89005-1018 702-435-7947 [email protected] www.padrak.com/vesperman TABLE OF CONTENTS Title Page INTRODUCTION ............................................................................................................. 1 BRIEF SUMMARIES ....................................................................................................... 2 LARGE GENERATORS ............................................................................................... 13 Hydro-Magnetic Dynamo ............................................................................................ 13 Focus Fusion ............................................................................................................... 19 BlackLight Power’s Hydrino Generator ..................................................................... 19 IPMS Thorium Energy Accumulator .......................................................................... 22 Thorium Power Pack ................................................................................................... 22 Magneto-Gravitational Converter (Searl Effect Generator) ..................................... 23 Davis Tidal Turbine ..................................................................................................... 25 Magnatron – Light-Activated Cold Fusion Magnetic Motor ..................................... 26 Wireless Power and Free Energy from Ambient -

Indication of Anomalous Heat Energy Production in a Reactor Device Containing Hydrogen Loaded Nickel Powder
Indication of anomalous heat energy production in a reactor device containing hydrogen loaded nickel powder. Giuseppe Levi Bologna University, Bologna, Italy Evelyn Foschi Bologna, Italy Torbjörn Hartman, Bo Höistad, Roland Pettersson and Lars Tegnér Uppsala University, Uppsala, Sweden Hanno Essén Royal Institute of Technology, Stockholm, Sweden ABSTRACT An experimental investigation of possible anomalous heat production in a special type of reactor tube named E-Cat HT is carried out. The reactor tube is charged with a small amount of hydrogen loaded nickel powder plus some additives. The reaction is primarily initiated by heat from resistor coils inside the reactor tube. Measurement of the produced heat was performed with high-resolution thermal imaging cameras, recording data every second from the hot reactor tube. The measurements of electrical power input were performed with a large bandwidth three- phase power analyzer. Data were collected in two experimental runs lasting 96 and 116 hours, respectively. An anomalous heat production was indicated in both experiments. The 116-hour experiment also included a calibration of the experimental set-up without the active charge present in the E-Cat HT. In this case, no extra heat was generated beyond the expected heat from the electric input. Computed volumetric and gravimetric energy densities were found to be far above those of any known chemical source. Even by the most conservative assumptions as to the errors in the measurements, the result is still one order of magnitude greater than conventional energy sources. INTRODUCTION Andrea Rossi claims to have invented an apparatus that can produce much more energy per unit weight of fuel than can be obtained from known chemical processes. -

Nuclear Reactions in Micro/Nano-Scale Metal Particles
Few-Body Syst (2013) 54:25–30 DOI 10.1007/s00601-012-0374-6 Y. E. Kim Nuclear Reactions in Micro/Nano-Scale Metal Particles Received: 20 September 2011 / Accepted: 24 December 2011 / Published online: 17 March 2012 © Springer-Verlag 2012 Abstract Low-energy nuclear reactions in micro/nano-scale metal particles are described based on the the- ory of Bose–Einstein condensation nuclear fusion (BECNF). The BECNF theory is based on a single basic assumption capable of explaining the observed LENR phenomena; deuterons in metals undergo Bose–Einstein condensation. The BECNF theory is also a quantitative predictive physical theory. Experimental tests of the basic assumption and theoretical predictions are proposed. Potential application to energy generation by igni- tion at low temperatures is described. Generalized theory of BECNF is used to carry out theoretical analyses of recently reported experimental results for hydrogen–nickel system. 1 Introduction Over the last two decades, there have been many publications reporting experimental observations of excess heat generation and anomalous nuclear reactions occurring in metals at ultra-low energies, now known as low-energy nuclear reactions (LENR). Theoretical explanations of the LENR phenomena will be described based on the theory of Bose-Einstein condensation nuclear fusion (BECNF) in micro/nano-scale metal parti- cles [1–3]. The BECNF theory is based on a single basic assumption capable of explaining the observed LENR phenomena; deuterons in metals undergo Bose–Einstein condensation. While the BECNF theory is able to make general qualitative predictions concerning LENR phenomena it is also a quantitative predictive physical theory. Proposed experimental tests of the basic assumption and theoretical predictions will be described. -

Secrets of E-Cat
Mario Menichella SECRETS OF E-CAT Consulente Energia Index PREFACE ……….……………………………………………………………………. p. 5 1. WHAT E-CAT IS What are low-energy nuclear reactions, p. 10 – Twenty years of research on cold fusion, p. 12 – The disruptive innovations brought by Andrea Rossi, p. 15 – The findings of a “skeptic” to the presentation of E-Cat, p. 18. 2. HOW MUCH ENERGY IT PRODUCES The first methods used to calculate the excess energy, p. 24 – The early experimental results obtained, p. 26 – The public demonstrations of the E- Cat, p. 30 – Uncertainties related to the amplification factor, p. 34. 3. HOW IS AN E-CAT MADE? The architecture of the commercial version, p. 41 – What are the internal components of the E-Cat, p. 43 – How the external part of the E-Cat is made, p. 46 – A reconstruction of the heart of the reactor, p. 48. 4. DISCOVERING THE SETUP The source of hydrogen and its pressure, p. 54 – The ignition temperature of the nuclear reaction, p. 57 – Nickel powder: the quantity and the ideal size, p. 61 – The isotopic composition and metal treatment, p. 64 – A simple list of what you need, p. 67. 5. THE SECRET CATALYST The importance of an “additive” in the E-Cat of Rossi-Focardi, p. 71 – What is the function of the catalyst in a reaction?, p. 74 – The possibility that it may not be a compound, p. 77 – Some valuable and… totally unexpected clues, p. 80 – What are the conclusions that can be drawn?, p. 84. 6. PRODUCTS OF THE REACTIONS The substances observed in the post-reaction powder, p. -

How Can 30% of Nickel in Rossi's Reactor Be Transmuted Into Copper
How can 30% of nickel in Rossi’s reactor be transmuted into copper? « J... http://www.journal-of-nuclear-physics.com/?p=473&cpage=7#comments « From the orbital capture to some types of Introduction to quantum ring theory » L.E.N.R. reactions How can 30% of nickel in Rossi’s reactor be transmuted into copper? by Dott. Giuliano Bettini Retired. Earlier: Selenia SpA, Rome and IDS SpA, Pisa Also Adjunct Professor at the University of Pisa Adjunct Professor at Naval Academy, Leghorn (Italian Navy) Abstract In the present article I would like to answer a question posed by L. Kowalsky in a recent paper: how can 30% of nickel in Rossi’s reactor be transmuted into copper? “Everything should be made as simple as possible, but not simpler”, says a guy. I apologizes if I am too simplistic here. Introduction The interest on Andrea Rossi’s Nickel-Hydrogen Cold Fusion technology is accelerating [1]. However, Rossi says that about 30% of nickel was turned into copper, after 6 months of uninterrupted operation. Kowalski [2]. says that “this seems to be impossible because the produced copper isotopes rapidly decay into Ni”. But how it works? How it works Following Focardi Rossi [3]. a Ni58 nucleus produces a Copper nucleus according to the reaction Ni58 + p → Cu59 Copper nucleus Cu59 decays with positron (e+) and neutrino (ν) emission in Ni59 nucleus according to Cu59 → Ni59 + ν + e+ Then (e+) annichilates with (e-) in two gamma-rays e- + e+ → γ + γ Starting [3] from Ni58 which is the more abundant isotope, we can obtain as described in the two above processes Copper formation and its successive decay in Nickel, producing Ni59, Ni60, Ni61 and Ni62. -

Review Article Future Green Energy Through Cold Fusion Nuclear Heat
Scholars Journal of Engineering and Technology (SJET) ISSN 2321-435X (Online) Sch. J. Eng. Tech., 2014; 2(6B):913-917 ISSN 2347-9523 (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Review Article Future Green Energy Through Cold Fusion Nuclear Heat With Nickel Powder U. V. S. Seshavatharam1, S. Lakshminarayana2 1Honorary faculty, I-SERVE, Alakapuri, Hyderabad-35, AP, India 2Dept. of Nuclear Physics, Andhra University, Visakhapatnam-03, AP, India *Corresponding author U. V. S. Seshavatharam Email: Abstract: Based on the principle of conservation of energy and from the well known nuclear fusion and fission reactions it is possible to guess that, the E-CAT hidden energy may be in the form of binding of protons and neutrons of the Nickel, Hydrogen and Lithium atomic nuclei. In view of the recently developed compact 1MW E-CAT power plant designed by the Leonardo corporation, Nickel can certainly be considered as the ultimate substitute of Coal, Oil and Uranium in near future. Keywords: Cold Fusion, Low temperature nuclear reactions, E-CAT ( Energy Catalyze) INTRODUCTION With reference to the current [1-6] and old [7] physics in the near future. Andrea Rossi says: “one review reports, one can understand the current „golden gram of Nickel is equivalent to 5,00,000 liters of oil”. status‟ and old „Pathetic status‟ of Cold fusion or Low As there is no emission of the alpha, beta and gamma energy nuclear interactions (LENR). Since 1989 many radiation, as there is no emission of other gasses like scientists proposed many interesting proposals for SO2,CO2 etc, as Nickel is abundance is a high in the understanding the observed excess heat generation with earth core and as the technical risk of power generation various experimental setups[8-18]. -

June 17, 2016 Please Insert This Comment in the Record of the June
June 17, 2016 Please insert this comment in the record of the June 17, 2016 meeting of the Nevada Legislative Energy Committee: The Nevada Legislative Energy Committee typically focuses on conventional energy technologies such as solar, wind, geothermal and fossil fuels. The Committee should also learn about numerous alternative energy innovations and their potentially significant economic and environmental advantages over mainstream energy technologies. The Alexander Library in North Las Vegas is currently hosting part of my exhibit “Gallery of Clean Energy Inventions”. It displays summaries of 44 clean electricity generators, 20 advanced self-powered electric vehicle innovations, 26 radioactivity neutralization methods, 24 space travel innovations, 14 technical solutions to water shortages, and my own design of a torsion field school network. For a copy plus approximately 1000 pages of invention descriptions visit my website padrak.com/vesperman. For example, a doughnut-shaped hydro-magnetic dynamo the size of a two-car garage apparently could generate half of Hoover Dam’s 2080 megawatts without fuel nor pollution. Included below is my two-page list of “Clean Energy Inventions”. Attached is inventor David Yurth’s concept proposal for a “Technology R & D Innovation Center”. He presents a thoughtful plan for proactively discovering, evaluating, developing and commercializing new energy technologies. Included are his summaries of seven ‘bridge technologies’ such as for example electronically shaded glass and a conical vortex heat exchange engine. He also includes summaries of eight ‘advanced technologies’ such as thin film power generating disks and self-recharging gel cells. Gary Vesperman 588 Lake Huron Lane Boulder City, NV 89005-1018 702-435-7947 [email protected] Agenda Item XII D - ENERGY Meeting Date: 06-17-16 Clean Energy Inventions A portfolio of disruptive inventions has been accumulated after more than two decades of research and collaboration with numerous inventors, a few of whom are among the world’s most productive.